>Corresponding Author : Nooli Nishank
>Article Type : Case Report
>Volume : 2 | Issue : 1
>Received Date : 12 March, 2022
>Accepted Date : 27 March, 2022
>Published Date : 30 March, 2022
>DOI : https://doi.org/10.54289/JAAD2200104
>Citation : Mitra B, Andrew R, Daniel A, Harold M, Nishank N. (2022) Peri-Operative Management of Patients with Continuous Alcohol Monitoring Devices: A Case Report and Review of Literature. J Anaesth Anesth Drug 2(1): doi https://doi.org/10.54289/JAAD2200104
>Copyright : © 2022 Mitra B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access
1Department of Anesthesiology and Perioperative Medicine, Wayne State University School of Medicine, Detroit, Michigan
2Department of Anesthesiology and Perioperative Medicine, Saint Joseph Mercy Oakland Hospital, Pontiac, Michigan
*Corresponding author: Nooli Nishank, Wayne State University School of Medicine, Detroit, Michigan
Abstract
Continuous alcohol monitoring devices are worn among offenders who have been referred by the court and are designed to keep track of alcohol consumption. Also, named transdermal alcohol sensors, these devices measure the concentration of alcohol in perspiration every 30 minutes and can give a continuous estimation of alcohol levels over time [1]. Notably, very little prospective data exists to guide the management of a patient with a monitoring device in a surgical case that necessitates the use of monopolar or bipolar electrocautery. We encountered a patient who had chosen to undergo an elective robotic hysterectomy, and it was not until the patient reached the operating room that the continuous alcohol monitoring system was discovered on her ankle. Prior to the surgery, several layers of gauze were packed between the device and the patient’s skin, and a grounding pad was placed on the contralateral buttock. No adverse outcomes were encountered during the operation and the patient was safely discharged.
Keywords:Continuous alcohol monitoring device, Electrocautery, SCRAM device
Introduction
For alcohol offenders, such as individuals with multiple driving under the influence (DUI) convictions, an option for monitoring drinking behaviour is the use of court-ordered continuous alcohol monitoring devices. Specifically, one design is in the form of an ankle bracelet which is worn for a pre-determined period and cannot be removed by the individual. This device is called the SCRAM (Secure Continuous Remote Alcohol Monitor) - it contains an electrochemical alcohol sensor as well as sensors that measure skin temperature and skin contact; this data can then be transferred to a secure server to allow for a supervising individual to access the information [1].
Electrocautery is used routinely during surgery for tissue dissection and hemostasis. There are two kinds of electrosurgical units, including Bipolar and Monopolar. Contrary to monopolar electrocautery, in which the current passes through the patient to a return electrode pad, the bipolar electrocautery device functions as both the active and return electrode [2]. In other words, the current only passes through tissue in between the forceps and very little electrical current flows through the patient. For this reason, there is no requirement for a return electrode grounding pad. Inherently, however, electrocautery devices can be a safety hazard and have been known to interfere with other electrical equipment. More so with monopolar electrocautery, the patient should be positioned and insulated so that there is no skin contact with grounded metal objects and jewellery should be removed [2]. We encountered a patient who presented for an elective surgery involving electrocautery and, in the operating room, she was found to be wearing a continuous alcohol monitoring ankle bracelet.
Case Presentation
We are reporting a case involving a 63-year-old female who presented for an elective robotic hysterectomy due to menorrhagia and abnormal uterine bleeding. In reviewing the patient’s chart, the question of alcohol use had been asked and the patient had replied that she was a former drinker; however, there was lack of communication of the existence of ankle bracelet from the preoperative team to the intraoperative team and lack of documentation noting the alcohol monitor on her ankle. The device was noticed in the operating room (OR) following intubation (Figure 1). The surgical case was to be performed using electrocautery; therefore, measures were taken to prevent interference with the patient’s ankle monitor. A thick layer of Kerlix gauze was placed between the patient’s skin and the device, which potentially will insulate the patient from the existing metal component on the inner side of the SCRAM bracelet. As the patient was required to be in lithotomy position, a large foam pad was placed between the device and leg stirrup to avoid any pressure related injury (Figure 2). After discussing with the surgical team plan was made to avoid using monopolar cautery and instead use bipolar electrocautery device to minimize any electrical interference with the SCRAM device. Though a grounding pad is not required for bipolar electrocautery, one was placed on the contralateral buttock for extra safety precaution. There were no adverse outcomes during the case, and no injury was noted on the patient’s ankle following the operation.
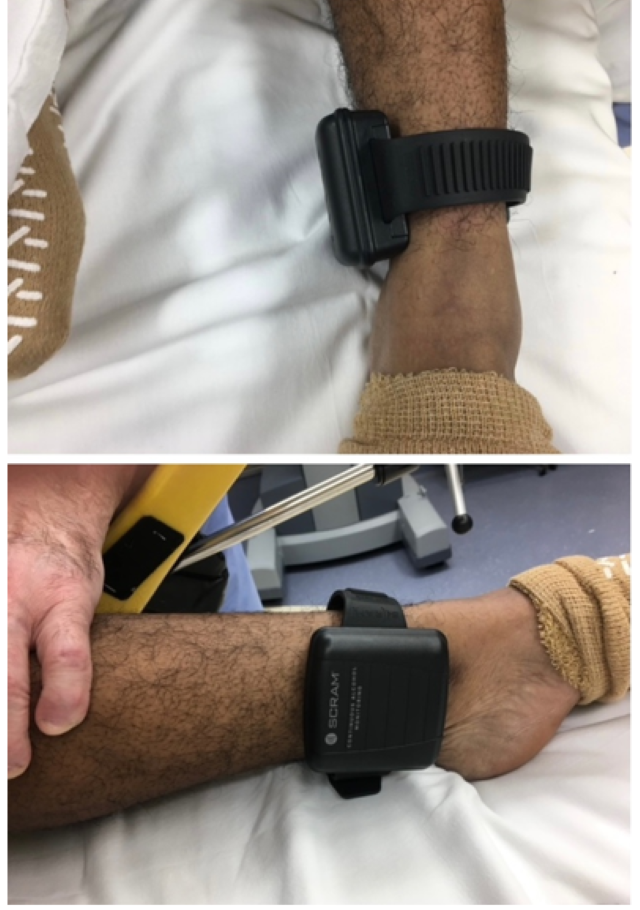
Figure 1: Continuous alcohol monitoring device (SCRAM) seen around the patient’s ankle.
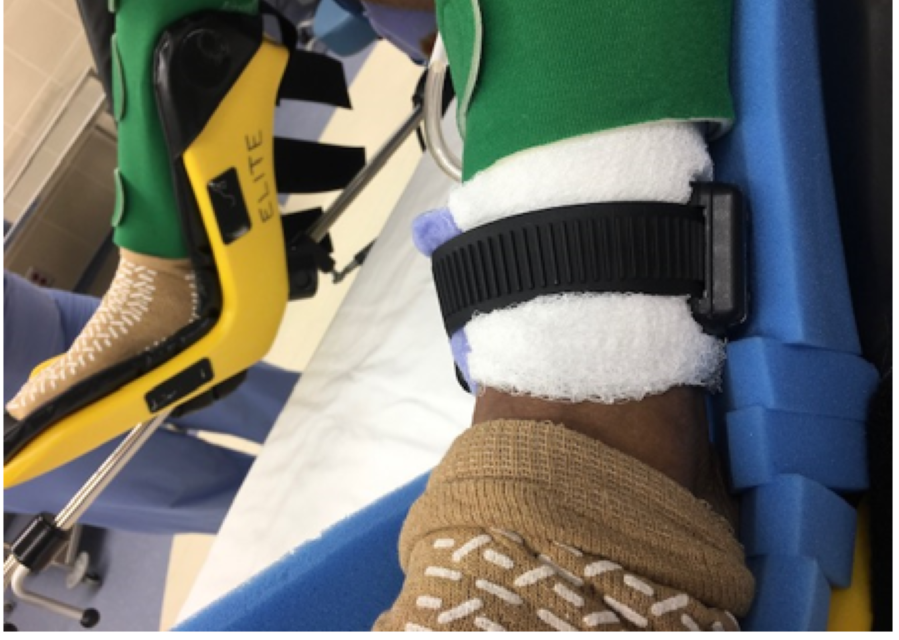
Figure 2: Patient’s legs in stirrups showing Kerlix wrapped around the patient’s left ankle separating the SCRAM device from skin surface and an extra foam pad used to insulate SCRAM device from left leg stirrup.
Discussion
Certain individuals who have had altercations with the law due to alcohol use, such as those with multiple DUI convictions, may be court-ordered to wear a monitoring device on their wrist or ankle. These devices track alcohol levels over time and allow for supervision of alcohol abstinence by courts or authorities. As common as these devices may be in the criminal justice system, very few guidelines exist on how to manage electrosurgical cases for these patients.
The only reported case found on literature review highlights the approach taken when 29-year-old gentlemen undergoing bilateral cranioplasty was noticed to have an electronic home monitoring device on his ankle [3]. Monopolar electrocautery was to be used for the case, and to prevent electrical interference with the ankle device, the grounding pad was placed on the contralateral thigh, gauzes were placed between the skin and the device, and the electrocautery was set at a low frequency (20 MHz). In our case we employed an extra measure of safety by using bipolar electrocautery in addition to taking similar precautions which included (1) a thick layer of kerlix gauze between the patient’s skin and the device, (2) placement of the return electrode pad on the contralateral buttock and (3) padding between the device and the leg stirrups due to lithotomy positioning. The patient did well during surgery and no adverse outcomes were noted prior to discharge. One month following the procedure, the patient denied any negative events and reported normal function of her alcohol monitoring device.
Consent for publication: A written informed consent was obtained from the patient for publication of clinical details and necessary images.
Conflicts of interest: All the authors do not have any conflicts of interest to disclose
References
- Leffingwell TR, Cooney NJ, Murphy JG, et al. (2013) Continuous Objective Monitoring of Alcohol Use: Twenty-First Century Measurement Using Transdermal Sensors. Alcoholism: Clinical and Experimental Research. 37(1): 16-22. [PubMed.]
- Cordero I. (2015) Electrosurgical units - how they work and how to use them safely. Community Eye Health. 28(89): 15-16. [Ref.]
- Sarpong Y, Dagal A, Sharar S, Avellino A. (2010) Safety of Electronic Home Monitoring Devices in the Operating Room. The Internet Journal of Anesthesiology. Volume 29 Number 1. [Ref.]
