>Corresponding Author : Benmalek Rime
>Article Type : Case Report
>Volume : 2 | Issue : 1
>Received Date : 20 May, 2022
>Accepted Date : 30 May, 2022
>Published Date : 02 June, 2022
>DOI : https://doi.org/10.54289/JCRMH2200105
>Citation : Benmalek R, El Abasse Z, Bendahou H, Asklou A, Adaoui A, et al. (2022) Preexcitation with Multiple Accessory Pathways as a First Presentation of Undiagnosed Late Stage Ebstein’s Anomaly in an Adult. J Case Rep Med Hist 2(1): doi https://doi.org/10.54289/JCRMH2200105
>Copyright : © 2022 Benmalek R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access
Cardiology department, Ibn Rochd University Hospital, Casablanca, Morocco
*Corresponding author: Benmalek Rime, Department of cardiology, University Hospital Center Ibn rochd, Casablanca, Morocco
Abstract
Ebstein’s anomaly (EA) is a relatively rare congenital heart disease that has long been a challenge both to cardiac surgeons and electrophysiologists. In addition to the hemodynamic burden of the tricuspid valve defect itself, the electrocardiogram (ECG) is abnormal in most cases with a high incidence of tachyarrhythmias, that can often be imputable to accessory atrioventricular pathways mostly located along the tricuspid posterior and septal valve leaflets, and rarely, to multiple accessory pathways. We here report the case of a 19-year-old girl with no known cardiac history, in whom a late stage EA with Eisenmenger syndrome, was diagnosed following a syncopal episode attributed to preexcited atrial tachy-fibrillation with an aspect consistant with multiple accessory pathways with at least one of them being right postero-septal. The patient’s management was very challenging for the heart team considering the fixed pulmonary hypertension and the fact she was reluctant to catheter ablation.
This case report underlines the importance of early diagnosis and treatment of EA and its rhythmic complications and explores the anatomic peculiarities of this disease with attention to features that could be important to both arrhythmogenesis and ablation therapy in this unique population.
Keywords: Ebstein’s Anomaly; Preexcitation; Multiple Accessory Pathways
Abbreviations: EA: Ebstein’s Anomaly, ECG: Electrocardiogram, AF: Atrial Fibrillation, Mpap: Mean Pulmonary Arterial Pressure, Pvri: Pulmonary Vascular Resistance Index, RV: Right Ventricle, ASD: Atrial Septal Defect, MRI: Magnetic Resonance Imaging, RF: Radiofrequency, AVRT: Atrioventricular Re-Entry Tachycardia
Introduction
Ebstein's anomaly (EA) is a rare congenital condition representing less than 1% of congenital heart disease [1] in which there is downward displacement of insertion of septal and posterior tricuspid valve leaflets which are usually are dysplastic. Anterior leaflet is not usually affected. This resulsts in poor coaptation of the valve leaflets leading to tricuspid regurgitation, and therefore to atrial enlargement of a variable degree depending upon the degree of tricuspid regurgitation. The clinical presentation varies depending on the period of discovery, ranging from the very serious neonatal form to better tolerated forms in adolescents and adults [1]. The abnormal development of the tricuspid valve described in EA results in several activation abnormalities, including intraatrial conduction delay, right bundle branch block, and ventricular preexcitation [2]. We report the case of an adult diagnosed with Ebstein's disease after an atrial tachy-fibrillation type rhythm disorder with preexcitation.
Case Report
We report the case of a 19-year-old girl with no known cardiac history, who presented to the Emergency department for a syncopal episode associated with an increasing exertional dyspnea and palpitations. On admission, the patient was tachypneic with cold extremities, lips cyanosis and hypotension at 79/42 mmHg, tachycardia at 187 beats/min and oxygen saturation of 89%.
Her electrocardiogram (ECG) showed atrial fibrillation (AF) at 190 beats/min with QRS duration 128 ms (Figure 1). The patient immediately received Electrical Synchronized Cardioversion of 150 joules with restoration of sinus rhythm at 75 beats/min with an aspect consistant with multiple accessory pathways with at least one of them being right postero-septal (Figure 2). Chest radiography showed a globe-shaped heart with cardiothoracic ratio of 0.65. Echocardiography found severe right heart enlargement with large atrialization of the right ventricle and apical displacement of the tricuspid valve consistent with EA, associated with an ostium secondum Atrial Septal Defect of 27 mm with right-to-left shunt and a severe tricuspid regurgitation (Figure 3). The patient underwent right heart catheterization that showed the following results : Mean Pulmonary Arterial Pressure (Mpap)= 59 mmHg, Pulmonary Vascular Resistance Index (PVRi)= 10,3 WU.m², shunt ratio Qp/Qs= 2,46, and there was no significant decrease in PVRi after Vasodilator testing using 100% oxygen. The patient’s case was discussed by the heart team, and given the Eisenmenger syndrome, the surgical closure of her ASD was not considered, and medical treatment with sildenafil was initiated. As for her arrhythmia, she was proposed for an electrophysiological study and catheter ablation procedure of her accessory pathways but the patient refused to undergo the procedure, and was discharged from hospital under bisoprolol after concertation with the rythomolgy team, in addition to oral anticoagulation with Acenocoumarol, and was closely followed-up as an out-patient since then.
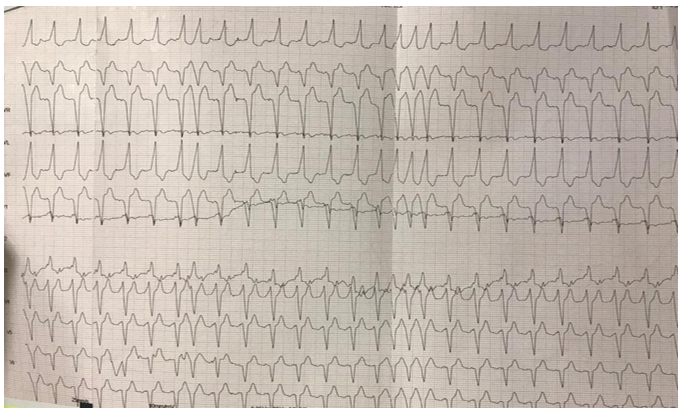
Figure 1: 12-leads ECG at presentation, showing preexcitated AF at 190 beats/min with wide QRS and suggesting multiple accessory pathways.
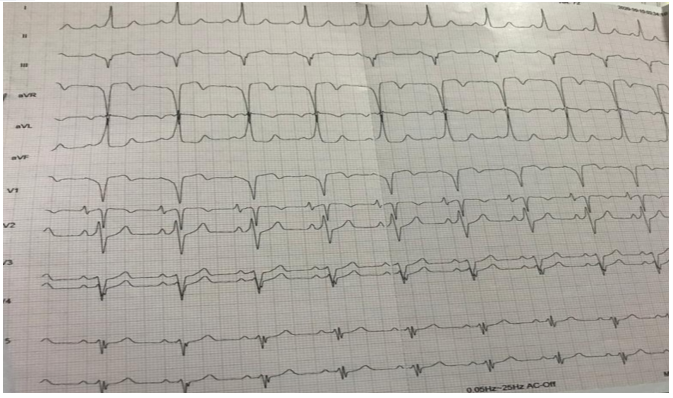
Figure 2: The patient’s ECG after she received 150 Joules Electrical Synchronized Cardioversion showing restoration of sinus rhythm at 75 beats/min.
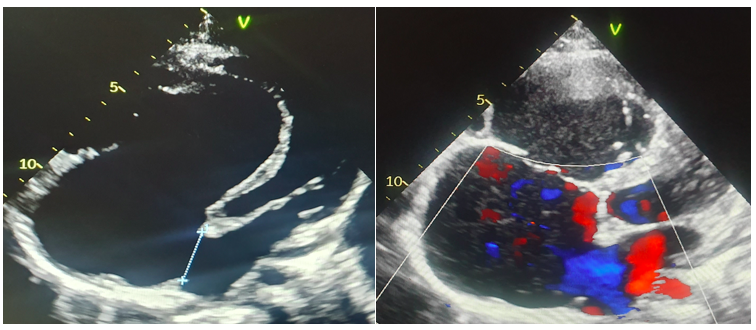
Figure 3: Transthoracic echocardiography with 4 chambers apical view showing Ebstein’s anomaly, associated with a 27 mm ASD with right-to-left shunt and a severe TR.
Discussion:
Ebstein's disease is a rare congenital heart disease, with several clinical and anatomical presentations. It can be revealed at birth by a severe neonatal respiratory distress, as it can first present at a late stage as right heart failure and/or a supraventricular arrhythmia in adolescents and adults.
There are four main features in EA:
1. Apical displacement of the tricuspid valve in the right ventricle with valve distortion and tricuspid regurgitation;
2. Enlarged right heart chambers, especially the right atrium;
3. Atrial communication which is present in 49 to 75% of patients;
4. And arrhythmias, in particular atrial fibrillation (AF), usually with preexcitation.
Electrocardiogram (ECG) findings:
The ECG is abnormal in most patients with EA. It may show high and wide P waves as a result of right atrial enlargement, as well as complete or incomplete right bundle branch block. R waves in leads V1 and V2 are small. Different morphologies of the QRS pattern reflecting infra-hisian conduction disorders [3]. Moreover, arrhythmias are known to occur frequently in patients with EA due both to congenital arrhythmogenic substrates and to acquired substrates due to post-cardiac surgery alterations. The arrhythmogenic potential of EA can already be guessed from the recorded surface ECG [4].
Atrioventricular blocks: Complete atrioventricular block is rare in EA, but first-degree AVB occurs in 42% of patients due to right atrial enlargement and structural abnormalities of the atrioventricular conduction system. The atrioventricular node may be compressed, and the central fibrous body may be abnormally formed as well as the right branch of the His bundle that can be fibrosed [3].
The downward displacement of the tricuspid septal leaflet is associated with a discontinuity of the central fibrous body and septal atrioventricular ring with direct muscle connections, which creates a potential substrate for accessory atrioventricular connections and preexcitation [3].
Tachyarrhythmias: Of all congenital heart defects, EA is most commonly associated with tachycardia. And this has a severe impact on the quality of life, morbidity and mortality [4].
Paroxysmal supraventricular tachyarrhythmias in EA are due to typical fast-conductive atrioventricular accessory pathways with anterograde and retrograde conduction properties in most patients, ectopic atrial tachycardia, atrial flutter, and AF can occur in 25 to 30 % of patients [4].
Depending on the series, 6% to 36% of patients with EA have an accessory pathway, and most of the accessory pathways are located around the orifice of the malformed tricuspid valve. Identifying and treating these accessory pathways is crucial in order to prevent sudden death [3].
Preexcitation syndrome: Although EA represents 1% of all congenital heart diseases, the prevalence of accessory pathways in this disease is much higher than in other congenital heart diseases [5].
The prevalence of the preexcitation pattern in EA ranges from 0.01% to 0.03%. Sudden cardiac death is often the first manifestation of the disease [6].
The apical displacement of the septal tricuspid leaflet is associated with the discontinuity of the central fibrous body and the septal atrioventricular ring. Part of the myocardium passes directly through the atrioventricular annulus in this position, thus forming a potential substrate for accessory atrioventricular connections and ventricular preexcitation.
Accessory pathways in EA are predominantly right with signs of ventricular pre-excitation and are located on the lower half of the tricuspid annulus with width variation [7].
AF and flutter: AF and common atrial flutter are usually secondary to structural alterations in the right atrial myocardium, such as fibrosis and dilatation.
The exact mechanism of AF is not yet well understood, while the evolution and critical components of the perpetuation of common-type atrial flutter have been explored in recent years [4].
The tachycardia follows its course along the tricuspid annulus, like a natural electrical conduction barrier, through a critical area of myocardial tissue closed off at the underside of the AV node by the insertion of the inferior vena cava, called the "posterior isthmus” [4].
Cardiac Imaging:
Echocardiography is the best tool for the diagnosis of EA enabling in most cases to avoid cardiac catheterization.
Echocardiography allows an accurate assessment of the leaflets of the tricuspid valve as well as the size and function of the heart chambers [4]. As a matter of fact, the comparison of 25 operated patients showed excellent concordance between echocardiographic and surgical findings [6].
Echocardiographic findings in EA show an apical shift of the posterior and septal tricuspid valve leaflets, exceeding 20 mm or 8 mm/m² in adults.
Consequently, the right heart is made of three components including the true right atrium, the functional right ventricle (RV) and an intermediate zone that is anatomically ventricular but functionally right atrial (atrialized RV). The thin wall of the atrialized RV can result in an aneurysm between the anatomical tricuspid ring and the apically displaced posterior leaflet. The annular attachment of the anterior leaflet is normal, it may be dysplastic and adherent to the right ventricular wall [7,8].
Tricuspid regurgitation is usually moderate to severe. The size, shape and function of the functional RV should be described. Paradoxical motion of the interventricular septum results in left ventricular geometry and function’s alterations.
Atrial septal defect (ASD) is frequently associated to these findings [8].
Echocardiographic findings in patients with EA are summed up in table 1.
RV ejection fraction can be assessed visually by echocardiography; but quantitative assessment is tricky because of the difficulty to assess the RV morphology in EA. Hence the usefulness of real-time three-dimensional echocardiography for RV quantification [6].
Intraoperative transesophageal echocardiography is important in the perioperative management of patients with EA because it can identify additional findings which can sometimes lead to important modifications of the surgical procedure [6].
Cine magnetic resonance imaging (MRI) can be used to assess ventricular size and function when echocardiographic image quality is not optimal.
For patients with EA, echocardiography and cardiac MRI data are complementary. Quantitative assessment of right chambers’ size and function is best done by cardiac MRI; however, identification of additional heart defects, valve anatomy, and criteras of repairability are best determined by echocardiography [6].
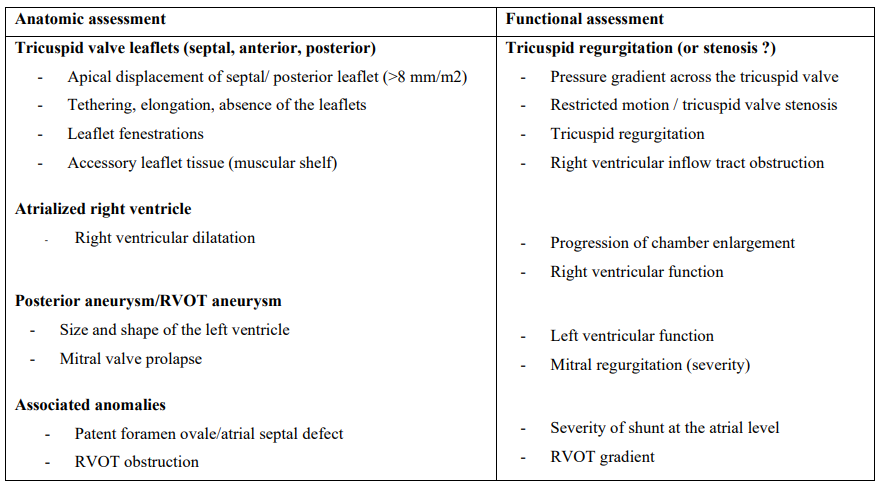
Table 1: Anatomic and functional assessment of patients with Ebstein’s anomaly [8]
Therapeutic management:
Asymptomatic patients may be managed medically with observation for a long period of time. Assessment of arrhythmias, progressive RV enlargement, and/or systolic RV dyfunction should be closely followed.
Patients should be considered for surgery when they develop symptoms and/or worsening exercise capacity, cyanosis, paradoxical embolism, progressive RV dilation or dysfunction, and the onset of arrhythmias and when tricuspid repair is feasible, with a low morbidity and mortality [9-10]. The surgical intervention mainly consists of tricuspid valve repair, RV plication, right atrial reduction, and atrial septal closure or subtotal closure.
While tricuspid repair is generally the goal, it should be emphasized that tricuspid valve replacement in adult patients show safer and more effective results [9]. Ventricular offloading with a bidirectional cavopulmonary shunt is performed selectively and generally reserved for cases of RV dysfunction [11].
Also, Arrhythmia surgery is advised with guidance provided by an electrophysiologist knowledgeable in EA. Catheter ablation with radiofrequency (RF) to interrupt an accessory atrioventricular connection is increasingly used as the primary treatment option for patients with re-entrant paroxysmal atrioventricular tachycardias. Although RF ablation in EA patients with symptomatic Wolf parkinson white or Atrioventricular Re-entry Tachycardia (AVRT) is a challenge due to structural malformations, it remains a very effective minimally invasive and curative therapy [5].
Conclusion
Ebstein's anomaly is a complex congenital anomaly with a broad anatomical and clinical spectrum. Its Management is complex and must be individualized. A precise knowledge of the different anatomical and hemodynamic variables, associated malformations and management options is essential. Thus, it is important that patients with EA are regularly followed by a cardiologist who specializes in congenital heart disease.
References
- Smith WM, et al. (1982) The Electrophysiologic Basis and Management of Symptomatic Recurrent Tachycardia in Patients with Ebstein’s Anomaly of the Tricuspid Valve. The American Journal of Cardiology. 49(5):1223-1234. [PubMed.]
- Torres PI. (2007) La anomalía de Ebstein asociada al síndrome de Wolff Parkinson-White. Arch Cardiol Mex. 77 Supl. 2 S2: 37-39. [PubMed.]
- Christine H, Jost MDA, Heidi M, Connolly MD, Joseph A, et al. (2007) Ebstein’s Anomaly Circulation. 115: 277-285. [Ref.]
- Hebe J, et al. (2000) Ebsteins Anomaly in Adults. Arrhythmias: Diagnosis and Therapeutic Approach Department Cardiology. Thorac Cardiov Surg. 48: 214-219. [PubMed.]
- Wei W, et al. (2014) Features of accessory pathways in adult Ebstein’s anomaly. Published on behalf of the European Society of Cardiology. Europace.16: 1619-1625. [PubMed.]
- Christine H, et al. (2012) Prospective comparison of echocardiography versus cardiac magnetic resonance imaging in patients with Ebstein’s anomaly. Int J Cardiovasc Imaging. 28: 1147-1159. [PubMed.]
- Sano S, Komori S, Amano T, Kohno I, Ishihara T, et al. (1998) Prevalence of ventricular preexcitation in Japanese school children. Heart. 79: 374-378. [Ref.]
- Oechslin E, Buchholz S, Ebsteins RJ. (2000) Anomaly in Adults: Doppler-Echocardiographic Evaluation. Echocardiography Laboratory, University Hospital, Zurich, Switzerland. Thorac Cardiov Surg. 48: 209-213. [PubMed.]
- Kimberly A, Holst MD, Heidi M, Connolly MD, et al. (2019) Ebstein's Anomaly. Cardiovasc J. 15(2): 138-144. [Ref.]
- Stout KK, Daniels CJ, Aboulhosn JA, et al. (2018) AHA/ACC Guideline for the Management of Adults with Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 139: e698-e800. [Ref.]
- Raju V, Dearani JA, Burkhart HM, et al. (2014) Right ventricular unloading for heart failure related to Ebstein's malformation. Ann Thorac Surg. 98(1): 167-173. [PubMed.]