>Corresponding Author : Gian Maria Pacifici
>Article Type : Mini Review
>Volume : 2 | Issue : 7
>Received Date : 04 Oct, 2022
>Accepted Date : 15 Oct, 2022
>Published Date : 18 Oct, 2022
>DOI : https://doi.org/10.54289/JCRMH2200128
>Citation : Pacifici GM. (2022) Clinical Pharmacology of Antituberculosis Drugs in Infants and Children. J Case Rep Med Hist 2(6): doi https://doi.org/10.54289/JCRMH2200128
>Copyright : © 2022 Pacifici GM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Mini Review | Open Access
Associate Professor of Pharmacology via Sant’Andrea 32, 56127 Pisa, Italy
*Corresponding author: Gian Maria Pacifici, Associate Professor of Pharmacology via Sant’Andrea 32, 56127 Pisa, Italy
Abstract
The antituberculosis drugs used in paediatric patients are: rifampicin, isoniazid, and pyrazinamide. The treatment of infections with rifampicin, isoniazid, and pyrazinamide has been reviewed. The efficacy and safety of rifampicin have been reported in children. The pharmacokinetics of rifampicin have been studied in infants and rifampicin median elimination half-life is 7.1 hours in infants with a postnatal age < 14 days and 3.5 hours in infants with a postnatal age ≥ 14 days. The treatment of children with rifampicin has been described. Rifampicin penetrates into the cerebrospinal fluid and treats the tuberculous meningitis and tuberculous meningo-encephalitis. Rifampicin is poorly transferred across the human placenta and poorly migrates into the breast-milk. The pharmacokinetics of isoniazid have been studied in children and the median elimination half-life of isoniazid is 3.22 hours. The treatment of children with isoniazid has been described and isoniazid poorly migrates into the breast-milk. The pharmacokinetics of pyrazinamide have been studied in children and the median elimination half-life of pyrazinamide is 3.47 hours and pyrazinamide poorly migrates into the breast-milk. The aim of this study is to review the treatment with rifampicin, isoniazid, and pyrazinamide. Rifampicin efficacy and safety, pharmacokinetics, treatment, penetration into the cerebrospinal fluid, treatments of meningitis, transfer across the human placenta, and migration into the breast milk have been reviewed. The efficacy and safety of isoniazid, the pharmacokinetics of isoniazid, and the migration of isoniazid into the breast-milk have been reviewed. The pharmacokinetics of pyrazinamide and the migration of pyrazinamide into the breast-milk have been reviewed.
Key words: Breast-Milk; Cerebrospinal-Fluid; Efficacy-Safety; Isoniazid; Meningitis; Pharmacokinetics; Placenta; Pyrazinamide; Rifampicin; Treatment
Abbreviations: POA: Pyrazinoic Acid, CSF: Cerebrospinal Fluid
Introduction
The antituberculosis drugs used in paediatric patients are: rifampicin, isoniazid, and pyrazinamide. The mechanism of action for rifampicin is typified by rifampicin action against Mycobacterium tuberculosis. Rifampicin enters bacilli in a concentration-depended manner, achieving steady-state concentrations within 15 min. Rifampicin binds to the β subunit of DNA-depended RNA polymerase to form a stable drug-enzyme complex. Drug binding suppress chain formation in RNA synthesis. Isoniazid enters bacilli by passive diffusion. The drug is not directly toxic to the bacillus but must be activated to its toxic form within the bacillus by catalase peroxidase (KatG), a multifunctional catalase-peroxidase. KatG catalyses the production from isoniazid of an isonicotinoyl radical that subsequently interacts with mycobacterial NAD and NADPD to produce a dozen adducts. One of these, a nicotinoyl-NAD isomer, inhibits the activities of enoyl acyl carrier protein reductase and β-ketoacyl-acyl carrier protein synthase. Inhibition of these enzymes inhibits synthesis of mycolic acid, an essential component of the mycobacterial cell wall, leading to bacterial cell death. Another adduct, a nicotinoyl-NADP isomer, potentially inhibits isoniazid (Ki < 1 nM) mycobacterial dihydrofolate reductase, thereby interfering with nucleic acid synthesis. Other products of KatG activation of isoniazid include superoxide H2O2, alkyl hydroperoxides, and the NO radical, which may also contribute to the mycobactericidal effect of isoniazid. Mycobacterium tuberculosis could be especially sensitive to damage from these radicals because the bacilli have a defect in the central regulator of the oxidative stress response, oxyR. Backup defences against radicals is provided by alkyl hydroperoxide reductase (encoded by ahpC), which detoxifies organic peroxides. Increased expression of ahC reduces isoniazid effectiveness. Pyrazinamide is activated by acidic conditions. Several mechanisms of action have been proposed. In one model, pyrazinamide passively diffuses into mycobacterial cells, in which Mycobacterium tuberculosis pyrazinamide (encoded by the pncA gene) deaminates pyrazinamide to pyrazinoic acid POA- (pyrazinoic acid) in its dissociated form which is then followed by passive diffusion of the POA- to the extracellular milieu. In an acidic extracellular milieu, a fraction of POA- is protonated to the unchanged form, POAH, a more lipid-soluble form that renters the bacillus and accumulates due to a deficient efflux pump. The Henderson-Hasselbalch equilibrium progressively favours the formation of POAH and its equilibration across membranes as the pH of the extracellular medium declines toward the pKa of pyrazinoic acid, 2.9. The acidification of the intracellular milieu is believed to inhibit enzyme function and collapse the transmembrane proton motive force, thereby killing the bacteria. Inhibitors of energy metabolism or reduced energy production states lead to enhanced pyrazinamide effect. A specific target of pyrazinamide has been proposed to be ribosomal protein S1 (encoded by RpsA) in the trans-translation process, so that toxic proteins due to stress accumulate and kill the bacteria. In addition, pyrazinamide’s target may include an aspartate decarboxylase (encoded by pnaD) involved in making precursors needed for pantothenate and CoA biosynthesis in persistent Mycobacterium tuberculosis [1].
Literature search
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “rifampicin children”, “isoniazid children” and “pyrazinamide children”. In addition, the book: The Pharmacological Basis of the Therapeutics [1] has been consulted.
Results
Rifampicin
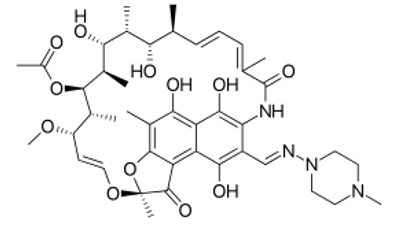
Figure 1: Rifampicin molecular structure (molecular weight = 822.94 grams/mole)
Administration of rifampicin to infants and children [2]
Oral administration or intravenous infusion of rifampicin to treat brucellosis in combination with other antibacterials, legionnaire’s disease in combination with other antibacterials, serious staphylococcal infections in combination with other antibacterials
Administration to newborns
Newborns. Give: 5 to 10 mg/kg twice-daily.
Administration to children
Children aged 1 to 11 months. Give: 5 to 10 mg/kg twice-daily.
Children aged 1 to 17 years. Give: 10 mg/kg twice-daily (maximum per dose = 600 mg twice-daily).
Oral administration of rifampicin to treat tuberculosis in combination with other drugs (intermittent supervised 6-mouth treatment) under expert supervision
Administration to children
Children. Give: 15 mg/kg thrice-daily (maximum per dose = 900 mg thrice-daily) for 6 months (initial and continuation phases).
Oral administration of rifampicin to treat tuberculosis in combination with other drugs (standard unsupervised 6-mounths treatment)
Treatment of children
Children with body-weight up to 50 kg. Give: 15 mg/kg once-daily for 6 months (initial and continuation phases); maximum = 450 mg once-daily) (initial and continuation phases).
Children with body-weight of 50 kg and above. Give: 15 mg/kg once-daily for 6 months (initial and continuation phases); maximum = 600 mg once-daily (initial and continuation phases).
Oral administration of rifampicin to treat congenital tuberculosis
Administration to newborns
Newborns. Give: 15 mg/kg once-daily for 6 months (initial and continuation phases).
Oral administration of rifampicin for the prevention of tuberculosis in susceptible close contacts or those who have become tuberculosis positive in combination with isoniazid
Administration to children
Children aged 1 month to 11 years with body-weight up to 50 kg.
Give: 15 mg/kg once-daily for 3 months (maximum = 450 mg once-daily) (initial and continuation phases).
Children aged 1 month to 11 years with body-weight of 50 kg and above. Give: 15 mg/kg once-daily for 3 months (maximum 600 mg once-daily) (initial and continuation phases).
Children aged 12 to 17 years with body-weight up to 50 kg. Give: 450 mg once-daily for 3 months.
Children aged 12 to 17 years with body weight of 50 kg and above. Give: 600 mg once-daily for 3 months.
Oral administration of rifampicin for the prevention of tuberculosis in susceptible close contacts or those who have become tuberculosis positive or who are isoniazid-resistant
Administration to children
Children aged 1 month to 11 years with body-weight up to 50 kg. Give: 15 mg/kg once-daily for 6 months (maximum = 450 mg once-daily).
Children aged 1 month to 11 years with body-weight of 50 kg and above. Give: 15 mg/kg once-daily for 6 months (maximum = 600 mg once-daily).
Children aged 12 to 17 years with body-weight up to 50 kg. Give: 450 mg once-daily for 6 months.
Children aged 12 to 17 years with body-weight of 50 kg and above. Give: 600 mg once-daily for 6 months.
Oral administration of rifampicin for the prevention of secondary case of Haemophilus influenzae type b disease
Administration to children
Children aged 1 to 2 months. Give: 10 mg/kg once-daily for 4 days.
Children aged 3 months to 11 years. Give: 20 mg/kg once-daily (maximum per dose = 600 mg once-daily) for 4 days.
Children aged 12 to 17 years. Give: 600 mg once-daily for 4 days.
Oral administration of rifampicin for the prevention of secondary case of meningococcal meningitis
Treatment of newborns
Newborns. Give: 5 mg/kg twice-daily for 2 days.
Treatment of children
Children aged 1 to 11 months. Give: 5 mg/kg twice-daily for 2 days.
Children aged 1 to 11 years. Give: 10 mg/kg twice-daily for 2 days.
Children aged 12 to 17 years. Give: 600 mg twice-daily for 2 days.
Oral administration of rifampicin to treat pruritus to cholestasis
Administration to children
Children. Give 5 to 10 mg/kg once-daily (maximum per dose = 600 mg once-daily).
Efficacy and safety of rifampicin in children
Treatment with rifampicin, at dose of 15 mg/kg once-daily for 4 months, effectively and safe treats tuberculosis in children [3]. Rifampicin, administered at a dose of 10 to 20 mg/kg once-daily, is safe and effective in ameliorating uncontrollable pruritus in children with persistent cholestasis [4].
Pharmacokinetics of rifampicin in infants
Smith et al. [5] studied the pharmacokinetics of rifampicin in 22 infants with a gestational age of 26 weeks (range, 23 to 41), and with postnatal age of 10 days (range, 0 to 84), and rifampicin was administered intravenously at a dose of 8 mg/kg once-daily in infants with a postnatal age < 14 days and at a dose of 15 mg/kg once-daily to infants with a postnatal age ≥ 14 days. Table 1 summarizes the pharmacokinetic parameters of rifampicin which have been obtained in these infants.
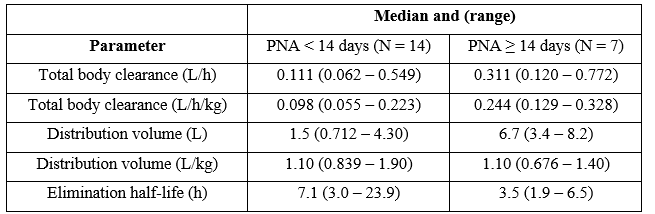
Table 1: Pharmacokinetic parameters of rifampicin which have been obtained in 22 infants. Values are the median and (range), by Smith et al. [5].
PNA = postnatal age.
This table shows that the total body clearance and the elimination half-life are dependent by the postnatal age. The median distribution volume is similar to the water volume and rifampicin is slowly eliminated. The median elimination half-life of rifampicin is double in infants with a postnatal age < 14 days than in infants with a postnatal age ≥ 14 days. Rifampicin is eliminated mainly by renal route and renal function increases with infant maturation. This consideration explains the longer elimination half-life in infants with a postnatal age of < 14 days than that in infants with a postnatal age ≥ 14 days. In addition, there is a remarkable interindividual variability in the pharmacokinetics and this variability is accounted by a wide variation of infant age and disease.
Treatment of children with rifampicin
Rifampicin, administered at a dose of 10 mg/kg once-daily, effectively and safe treats children with latent tuberculosis [6]. Rifampicin is more effective than placebo in the treatment of infection caused by Haemophilus influenzae type b [7]. Chemoprophylaxis, with a single once-daily dose of 15 mg/kg of rifampicin, effectively and safe treats leprosy in children [8].
Penetration of rifampicin in the cerebrospinal fluid (CSF)
In children with tuberculosis meningitis the concentration of rifampicin in the CSF is ≥ 0.5 µg/ml following the administration of rifampicin at a dose of 600 mg once-daily [9]. Rifampicin peak CSF concentrations, obtained 1 hour after the end of the infusion (600 mg once-daily), ranges from 0.57 to 1.24 µg/ml (median, 0.73 µg/ml) [10].
Treatment of meningitis with rifampicin
Rifampicin, administered at a dose of 600 mg once-daily, decreases the risk of mortality in children with tuberculous meningitis [11]. Rifampicin, administered at a dose of 20 mg/kg once-daily, effectively treats tuberculous meningitis [12]. Intraventricular rifampicin, administered at a dose of 20 mg/kg once-daily, is highly effective in treatment of severe tuberculous meningo-encephalitis [13].
Placental transfer of rifampicin
The transfer of rifampicin across the human placenta was studied using the perfusion of 7 placentas. The clearance indices of rifampin at maternal concentrations of 1.0 and 10.0 µg/ml are 0.12+05 and 0.12+0.11, respectively. Thus, rifampicin poorly crosses the human placenta [14].
Migration of rifampicin into the breast-milk
After a rifampicin single oral dose of 450 mg, the concentration of rifampicin in the milk ranges from 3.4 to 4.9 µg/ml. Thus, rifampicin poorly migrates into the breast-milk [15].
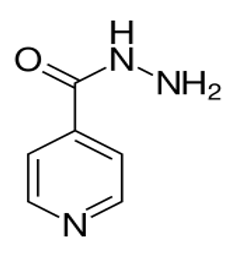
Isoniazid molecular structure (molecular weight = 137.139 grams/mole)
Administration of isoniazid to infants and children [16]
Oral administration or intravenous injection of isoniazid to treat tuberculosis in combination with other drugs (standard unsupervised 6-month treatment)
Administration to children
Children. Give: 10 mg/kg once-daily (maximum per dose = 300 mg once-daily for 6 months) (initial and continuation phases).
Oral administration or intravenous injection of isoniazid to treat tuberculosis in combination with other drugs (intermittent supervised 6-month treatment) under expert supervision
Administration to children
Children. Give: 15 mg/kg thrice-weekly (maximum per dose = 900 mg thrice-daily) for 6 months (initial and continuation phases).
Oral administration or intravenous injection of isoniazid to treat congenital tuberculosis in combination with other drugs
Administration to newborns
Newborns. Give: 10 mg/kg once-daily for 6 months (initial and continuation phases).
Oral administration or intramuscular injection or intravenous injection of isoniazid for the prevention of tuberculosis in susceptible close contacts or those who have become tuberculin positive
Administration to newborns
Newborns. Give: 10 mg/kg once-daily for 6 months (initial and continuation phases).
Administration to children
Children aged 1 month to 11 years. Give: orally 10 mg/kg once-daily (maximum per dose = 300 mg once-daily), alternatively give orally 10 mg/kg once-daily (maximum per dose = 300 mg once-daily) for 3 months, to be taken in combination with rifampicin.
Children aged 12 to 17 years. Give: 300 mg once-daily for 6 months, alternatively give 300 mg orally for 3 months, to be taken in combination with rifampicin.
Efficacy and safety of isoniazid in children
Isoniazid, administered at a dose of 10 mg/kg once-daily to children, effectively and safe treats tuberculosis [17]. Isoniazid, administered at a daily dose of 15 mg/kg, is safe and well-tolerated in HIV-infected children on concomitant antiretroviral therapy [18].
Pharmacokinetics of isoniazid in children
Phaisal et al. [19] studied the pharmacokinetics of isoniazid in 12 children aged 3.8 years (range, 2.1 to 4.9), weighing 16.4 kg (range, 10.2 to 29.0), and isoniazid was administered orally at a dose of 25 mg/kg once-daily (range, 21.7 to 26.8). Table 2 summarizes the pharmacokinetic parameter of isoniazid obtained in these children.
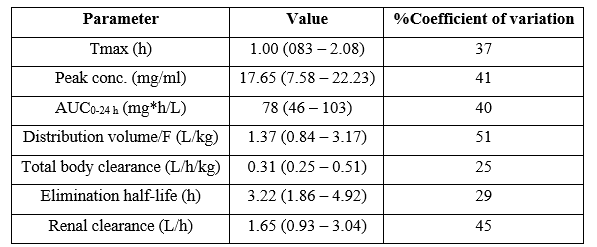
Table 2: Pharmacokinetic parameters of isoniazid which have been obtained in 12 children. Values are the median and (range), by Phaisal et al. [19]. Tmax = time to reach the peak concentration. AUC = area under the concentration-time curve. F = bioavailability.
This table shows that isoniazid is rapidly absorbed following oral dosing as the median time to reach the peak concentration is 1.0 hours, the median distribution volume of isoniazid is similar to the water volume, isoniazid is slowly eliminated as the median elimination half-life is 3.22 hours, and the renal clearance is higher than the total body clearance. In addition, there is a remarkable interindividual variability in the pharmacokinetic parameters and this variability is accounted by the wide variation in child age and disease.
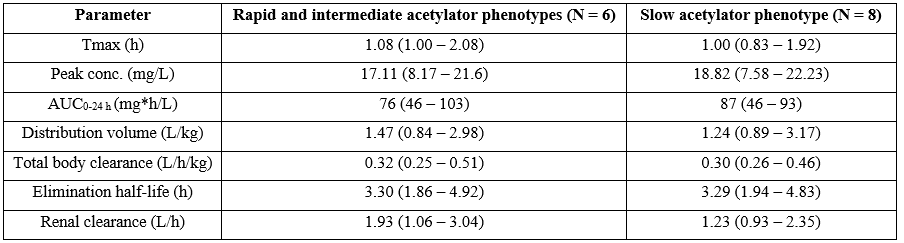
Table 3: Relationship between arylamine N-acetyltransferase 2 phenotype and isoniazid pharmacokinetic parameters. Values are the median and (range), by Phaisal et al. [19]. Tmax = time to reach the peak concentration. AUC = area under the concentration-time curve.
This table shows that isoniazid pharmacokinetic parameters are independent by arylamine N-acetyltransferase 2 phenotype.
Treatment of children with isoniazid
Isoniazid, administered at a dose of 15 mg/kg once-daily, reduces the risk of developing tuberculosis in children [20]. Younger children require higher doses of isoniazid per kilogram of body-weight to achieve isoniazid concentrations similar to those in adults and a daily isoniazid dose of 8 to 12 mg/kg is recommended in children [21]. Chemotherapy with isoniazid, administered at a dose of 20 mg/kg once-daily, successfully treats tuberculosis in children [22].
Migration of isoniazid into the breast-milk
A lactating woman was given an isoniazid single oral dose of 300 mg. Breast-milk was collected at various times during the next 24 hours. Peak isoniazid milk concentration of 16.6 µg/ml occurs at 3 hours after the dose and the half-life of isoniazid in milk is 5 hours [23]. Seven lactating women received isoniazid orally at a dose of 300 mg once-daily for at least 34 days. Blood and milk samples were collected at 0,1, 2, 3, and 4 hours after the dose and isoniazid peak concentration in milk ranges from 2 to 6.7 µg/ml which occurs 1 hour after the dose and isoniazid milk concentration drops by about half at 4 hours after the dose [24]. These results indicate that isoniazid poorly migrates into the breast-milk.
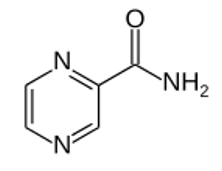
Pyrazinamide molecular structure (molecular weight = 123.113 grams/mole)
Administration of pyrazinamide to infants and children [25]
Oral administration of pyrazinamide to treat tuberculosis, in combination with other drugs (standard unsupervised 6-months treatment)
Administration to children
Children with body-weight up to 50 kg. Give: 35 mg/kg once-daily for 2 months (initial phase) (maximum = 1.5 grams once-daily).
Children with body-weight of 50 kg and above. Give: 35 mg/kg once-daily for 2 months (initial phase) (maximum = 2 grams once-daily).
Oral administration of pyrazinamide to treat tuberculosis, in combination with other drugs (intermittent supervised 6-months treatment) under expert supervision
Administration to children
Children with body-weight up to 50 kg. Give: 50 mg/kg
thrice-weekly (maximum per dose = 2 grams thrice-weekly)
thrice-weekly (maximum per dose = 2 grams thrice-weekly) for 2 months (initial phase).
Children with body-weight of 50 kg and above Give: 50 mg/kg thrice-weekly (maximum per dose = 2.5 grams thrice-weekly) for 2 months (initial phase).
Oral administration of pyrazinamide to treat congenital tuberculosis, in combination with other drugs
Administration to newborns
Newborns. Give: 35 mg/kg once daily for 2 months (initial phase).
Pharmacokinetics of pyrazinamide in children with tuberculosis
Zhu et al. [26] studied the pharmacokinetics of pyrazinamide in 21 children aged 4 years (range, 1.3 to 15.4) and weighting 16 kg (range, 8.5 to 59.0) and pyrazinamide was administered orally at a dose 250 mg (range, 125 to 2,000) once-daily or 1,125 mg (range, 750 to 1,500) thrice-weekly.
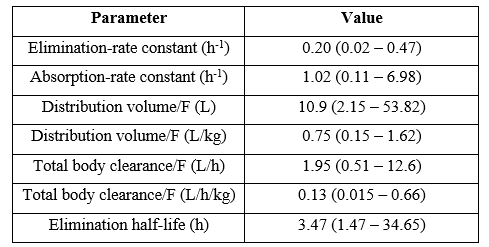
Table 4: Pharmacokinetic parameters of pyrazinamide which have been obtained in 21 children. Values are the median and (range), by Zhu et al. [26].
F = bioavailability.
This table shows that pyrazinamide is rapidly absorbed as the median absorption-rate constant in 1.02 h-1, the median distribution volume is similar to the water volume, and pyrazinamide is slowly eliminated as the median elimination-rate constant and the median elimination half-life are 0.20 h-1 and 3.47 hours, respectively.
Migration of pyrazinamide into the breast-milk
One lactating woman received 1 gram orally of pyrazinamide and the peak concentration of pyrazinamide in the milk is 1.5 µg/ml [27]. This result indicates that pyrazinamide poorly migrates into the breast-milk.
Discussion
The antituberculosis drugs used in paediatric patients are: rifampicin, isoniazid, and pyrazinamide. The treatment of infants and children with rifampicin, isoniazid, and pyrazinamide has been extensively reviewed. The efficacy and safety of rifampicin have been reported in children [3,4]. The pharmacokinetics of rifampicin have been studied in infants and the median elimination half-life is 7.1 hours in infants with a postnatal age < 14 days and 3.5 hours in infants with a postnatal age ≥ 14 days [5]. Rifampicin is eliminated mainly by renal route and the renal function improves with infant maturation. Rifampicin treats children with latent tuberculosis [6], children infected by Haemophilus influenzae type b [7], and children with leprosy [8]. Rifampicin penetrates into the cerebrospinal fluid [9,10] and treats tuberculosis meningitis [11,12] and tuberculous meningo-encephalitis [13]. Rifampicin is poorly transferred across the human placenta [14] and poorly migrates into the breast-milk [15]. The efficacy and safety of isoniazid have been described in children [17,18]. The pharmacokinetics of isoniazid have been studied in children and the median elimination half-life of isoniazid is 3.22 hours [19]. The treatment of children with isoniazid has been described [20-22], isoniazid, administered at a dose of 15 mg/kg once-daily, treats tuberculosis [20], younger children require higher doses of isoniazid than adults to achieve isoniazid concentrations similar to those in adults and a daily isoniazid dose of 8 to 12 mg/kg is recommended in children [21], isoniazid treats tuberculosis in children [22], and isoniazid poorly migrates into the breast-milk [23,24]. The pharmacokinetics of pyrazinamide have been studied in children with tuberculosis and the median elimination half-life is 3.47 hours [26], and pyrazinamide poorly migrates into the breast-milk [27].
In conclusion, the antituberculosis drugs used in paediatric patients are: rifampicin, isoniazid, and pyrazinamide. The treatment of children with rifampicin, isoniazid, and pyrazinamide has been extensively reviewed. The efficacy and safety of rifampicin have been reported in children. The pharmacokinetics of rifampicin have been studied in infants and the median elimination half-life of rifampicin is longer in children with a postnatal age < 14 days than in infants with a postnatal age ≥ 14 days. Rifampicin penetrates into the cerebrospinal fluid and treats tuberculous meningitis and tuberculous meningo-encephalitis. Rifampicin poorly crosses the human placenta and poorly migrates into the breast-milk. The efficacy and safety of isoniazid have been reported in children. The pharmacokinetics of isoniazid have been studied in children and the median elimination half-life of isoniazid is 3.22 hours, and isoniazid poorly migrates into the breast-milk. The pharmacokinetics of pyrazinamide have been studied in children with tuberculosis, the median elimination half-life of pyrazinamide is 3.47 hours, and pyrazinamide poorly migrates into the breast-milk. The aim of this study is to review the clinical pharmacology of rifampicin, isoniazid, and pyrazinamide in children.
Conflict of interests
The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria.
This article is a review and drugs have not been administered to men or animals.
Acknowledgments
The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.
References
- Gumbo T. (2018) “Chemotherapy of tuberculosis, Mycobacterium avium Complex Disease and Leprosy”. The Goodman & Gilman’s. The Pharmacological Basis of the Therapeutics, Brunton Hilal-dandan LL, Knollmann BC, editors, USA. 1066-1086. [Ref.]
- The British national formulary for children. (2019-2020) “Rifampicin”. Macmillan, 78th Edition, Hampshire International Business Park, Hampshire, Lime Three Way, Basingstoke, Hampshire, UK. 379-380. [Ref.]
- Diallo T, Adjobimey M, Ruslami R, Trajman A, Sow O, et al. (2018) Safety and side effects of rifampicin versus isoniazid in children. N Engl J Med. 379: 454-463. [PubMed.]
- El-Karaksy H, Mansour S, El-Sayed R, El-Raziky M, El-Koofy N, et al. (2007) Safety and efficacy of Rifampicin in children with cholestatic pruritus. Indian J Pediatr. 74(4): 279-281. [Ref.]
- Smith PB, Cotten M, Hudak ML, Sullivan JE, Poindexter BB, et al. (2019) Rifampin Pharmacokinetics and Safety in Preterm and Term Infants. Antimicrob Agents Chemother. 63(6): e00284-319. [PubMed.]
- Oh CE, Menzies D. (2022) Four months of rifampicin monotherapy for latent tuberculosis infection in children. Clin Exp Pediatr. 65(5): 214-221. [PubMed.]
- Murphy TV, Chrane DF, McCracken GH Jr, Nelson JD. (1983) Rifampin prophylaxis vs placebo for household contacts of children with Haemophilus influenzae type b disease. Am J Dis Child. 137(7): 627-632. [Ref.]
- dos Santos DS, Duppre NC, Sarno EN, Pinheiro RO, Sales AM, et al. (2018) Chemoprophylaxis of leprosy with rifampicin in contacts of multibacillary patients: study protocol for a randomized controlled trial. Trials. 19(4): 244-255. [Ref.]
- Mezochow A, Thakur KT, Zentner I, Subbian S, Kagan L, et al. (2019) Attainment of target rifampicin concentrations in cerebrospinal fluid during treatment of tuberculous meningitis. Int J Infect Dis. 84(7): 15-21. [Ref.]
- Nau R, Prange HW, Menck S, Kolenda H, Visser K, et al. (1992) Penetration of rifampicin into the cerebrospinal fluid of adults with uninflamed meninges. J Antimicrob Chemother. 29(6): 719-724. [PubMed.]
- Svensson EM, Dian S, Brake LT, Ganiem AR, Yunivita V. (2020) Model-Based Meta-analysis of Rifampicin Exposure and Mortality in Indonesian Tuberculous Meningitis Trials. Clin Infect Dis. 71(8): 1817-1823. [PubMed.]
- Wasserman S, Davis A, Stek C, Chirehwa M, Botha S, et al. (2021) Plasma Pharmacokinetics of High-Dose Oral versus Intravenous Rifampicin in Patients with Tuberculous Meningitis: a Randomized Controlled Trial. Antimicrobial Antimicrob Agents Chemother. 65(8): e0014021. [PubMed.]
- Vincken W, Meysman M, Verbeelen D, Lauwers S, D'Haens J. (1992) Intraventricular rifampicin in severe tuberculous meningo-encephalitis. Eur Respir J. 5(7): 891-893. [PubMed.]
- Magee KP, Wimberley D, Crane C, Sobhi S, Bawdon RE. (1996) Ex vivo human placental transfer of rifampin and rifabutin. Infect Dis Obstet Gynecol. 4(6): 319-322. [PubMed.]
- Lenzi E, Santaury S. (1969) Preliminary observations on the use of synthetic rifampicin derivative. Atti Academici Lancisaana Roma. 13(Suppl 1): 87-94. [Ref.]
- The British national formulary for children. (2019-2020) “Isoniazid”. Macmillan, 78th Edition, Hampshire International Business Park, Hampshire, Lime Three Way, Basingstoke, Hampshire, UK. 382. [Ref.]
- Hatzenbuehler LA, Starke R. (2014) Current Diagnosis and Treatment of Pediatric Latent Tuberculosis Infection. Current Pediatrics Reports. 2(6): 145-155. [Ref.]
- Gray DM, Workman LJ, Lombard CJ, Jennings T, Innes S, et al. (2014) Isoniazid preventive therapy in HIV-infected children on antiretroviral therapy: a pilot study. Int J Tuberc Lung Dis. 18(3): 322-327. [PubMed.]
- Phaisal W, Jantarabenjakul W, Wacharachaisurapol N, Tawan M, Puthanakit T, et al. (2022) Pharmacokinetics of isoniazid and rifapentine in young pediatric patients with latent tuberculosis infection. Int J Infect Dis. 122(9): 725-732. [PubMed.]
- Ayieko J, Abuogi L, Simchowitz B, Bukusi EA, Smith AH, Reingold A. (2014) Efficacy of isoniazid prophylactic therapy in prevention of tuberculosis in children: a meta-analysis. BMC Infect Dis. 14(2): 1-20. [Ref.]
- McIlleron H, Willemse M, Werely CJ, Hussey GD, Schaaf HS, et al. (2009) Isoniazid plasma concentrations in a cohort of South African children with tuberculosis: implications for international pediatric dosing guidelines. Clin Infect Dis. 48(11): 1547-1553. [PubMed.]
- Hsu KH. (1974) Isoniazid in the prevention and treatment of tuberculosis. A 20-year study of the effectiveness in children. JAMA. 229(5): 528-533. [PubMed.]
- Berlin CM, Lee C. (1979) Isoniazid and acetylisoniazid disposition in human milk, saliva and plasma. Fed Proc. 38(Part 1): 426-428. [Ref.]
- Singh N, Golani A, Patel Z, Maitra A. (2008) Transfer of isoniazid from circulation to breast milk in lactating women on chronic therapy for tuberculosis. Br J Clin Pharmacol. 65(3): 418-22. [Ref.]
- The British national formulary for children. (2019-2020) “Pyrazinamide”. Macmillan, 78th Edition, Hampshire International Business Park, Hampshire, Lime Three Way, Basingstoke, Hampshire, UK. 383. [Ref.]
- Zhu M, Starke JR, Burman WJ, Steiner P, Stambaugh JJ, et al. (2002) Population pharmacokinetic modeling of pyrazinamide in children and adults with tuberculosis. Pharmacotherapy. 22(6): 686-695. [PubMed.]
- Holdiness MR. (1984) Antituberculosis Drugs and Breast-feeding. Arch Intern Med. 144(9): 1888-1892. [PubMed.]