>Corresponding Author : Janaína Luz Narciso-Schiavon
>Article Type : Case Report
>Volume : 2 | Issue : 7
>Received Date : 10 Oct,2022
>Accepted Date : 27 Oct, 2022
>Published Date : 31 Oct, 2022
>DOI : https://doi.org/10.54289/JCRMH2200130
>Citation : Narciso-Schiavon JL, Da Silva J, Vieira DSC, Marins L, Prado PHT, et al. (2022) Duodenal Ulcers as Manifestation of Eosinophilic Gastroenteritis. J Case Rep Med Hist2(7): doihttps://doi.org/10.54289/JCRMH2200130
>Copyright : © 2022 Narciso-Schiavon JL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access
1Gastroenterology, Internal Medicine Department - Universidade Federal de Santa Catarina, Florianópolis, SC, Brasil
2Gastroenterology Division, Digestive System Unit, University Hospital - Universidade Federal de Santa Catarina, Florianópolis, SC, Brasil
3Allergy Division - University Hospital - Universidade Federal de Santa Catarina, Florianópolis, SC, Brasil
4Pathological Anatomy Division - University Hospital - Universidade Federal de Santa Catarina, Florianópolis, SC, Brasil
5Digestive Endoscopy, Digestive System Unit, University Hospital - Universidade Federal de Santa Catarina, Florianópolis, SC, Brasil
*Corresponding author: Janaína Luz Narciso Schiavon, Gastroenterology, Internal Medicine Department - Universidade Federal de Santa Catarina, Florianópolis, SC, Brasil and Gastroenterology Division, Digestive System Unit, University Hospital - Universidade Federal de Santa Catarina, Florianópolis, SC, Brasil
Abstract
The most common causes of duodenal ulcer are Helicobacter pylori infection and use of non-steroidal anti-inflammatory drugs (NSAIDs). We present a case of a patient with back pain, food impaction and nail dystrophy who exhibited eosinophilic esophagitis and duodenal ulcers via upper digestive endoscopy (UDE). Helicobacter pylori was not identified after repeated investigations with UDE and serology, and there was no healing of the ulcer with the use of a proton pump inhibitor after two years and suspension of NSAIDs. The etiology of the ulcer was identified by biopsy with the presence of eosinophils characterizing eosinophilic gastroenteritis. There was complete remission of the esophageal and duodenal symptoms, resolution of UDE lesions and normalization of the nails with oral prednisone 20mg for three months.
Keywords: Eosinophilic Esophagitis; Eosinophilic Enteropathy; Duodenal Ulcer; Abdominal Pain; Deglutition Disorders
Abbreviations: NSAID: Non-Steroidal Anti-Inflammatory Drugs, EG: Eosinophilic Gastroenteritis, UDE: Upper Digestive Endoscopy, PPI: Proton Pump Inhibitor, HPF: High Powered Field
Introduction:
Eosinophilic gastroenteritis (EG) is a rare disease with heterogeneity, characterized by the presence of intense infiltration of eosinophils in one or multiple segments of the gastrointestinal tract [1]. The pathogenesis of EG is still not well understood. There is strong evidence that EG is partly allergic in origin, including the findings in the literature that approximately 75% of patients with EG are atopic and that the aggressiveness of the disease can be mitigated with the use of an allergen-free diet [2]. The clinical manifestations of EG depend on the location and depth of eosinophil infiltration in the GI tract. The signs and symptoms vary according to the layer affected and may often overlap. The stomach and the small intestine are the main affected areas, but the stomach, the large intestine and the rectum are also greatly affected [3]. The presentation of duodenal ulcers is rarely described in the literature [4-15].
Case Report
A 27-year-old male complained of dysphagia due to dry solids and a previous history of food impaction that made him seek emergency care, which improved with vomiting. The individual always ate slowly and was usually the last person to leave the table. He had been taking anti-inflammatory medication for back pain over a long period of time. He had a history of allergic rhinitis and fish allergy. Physical examination was normal except for nail dystrophy in fingers. Upper digestive endoscopy showed eosinophilic esophagitis with suspected esophageal substenosis, mild enanthematous pangastritis, and two Sakita A1 duodenal ulcers (figure 1A, B). Anatomopathological examination revealed esophageal mucosa with 65 eosinophils/HPF, eosinophilic microabscesses-compatible with eosinophilic esophagitis. The gastric and duodenal mucosal biopsy were normal. The histologic research for Helicobacter pylori was negative. Esophageal contrast radiography was normal and showed no stenosis. Blood count and eosinophil count were normal. The patient was evaluated by the allergist who evidenced specific IgE test with moderate sensitivity for fish and low sensitivity for salmon and tuna. Prick test was positive for grass pollen (wild and cultivated), Blomia tropicalis, Dermatophagoides pteronyssinus, trees, mite storage, Tirophagus entomophagus, fish, corn and peanut. The patient was treated with a hypoallergenic diet, proton pump inhibitor (PPI) and swallowed corticosteroid. There was an improvement in the esophageal condition with the treatment after doubling the dose, with recurrence of the condition after discontinuation of treatment. The duodenal ulcer did not heal after two years of frequent clinical follow-up. Serology for Helicobacter pylori was negative. Differential diagnosis was conducted for the detection of several diseases. The nutritional profile was normal (normal values of ferritin, albumin, folic acid, vitamin B12) and the inflammatory tests were negative (erythrocyte sedimentation rate and C-reactive protein). Enterotomography showed thickening of a segment of the proximal jejunum loop with an extension of at least 8cm located medially to the inferior hepatic border and inferiorly to the duodenal bulb. Presence of prominent lymph nodes at the root of the mesentery next to the thickened segment without evidence of densification of the adjacent fat. Colonoscopy yielded normal results. A follow-up ulcer biopsy identified duodenal mucosa showing moderate mixed inflammatory, reparative epithelial changes and an area of erosion compatible with the edge of an active chronic ulcerated lesion, with 20 eosinophils/HPF (figure 1C). In addition, persistence of eosinophilic esophagitis was shown. After the diagnosis of EG, there was complete remission of the esophageal and duodenal symptoms with oral prednisone 20mg for three months, including normalization of the nails aspect (figure 1D). Two years after discontinuation of prednisone, the patient remains asymptomatic. Written informed consent was obtained from the patient.
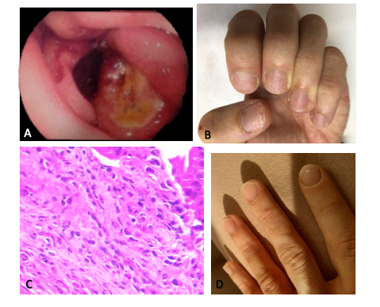
Figure 1: A) Upper Digestive Endoscopy showing two duodenal ulcers. B) Nail dystrophy. C) Anatomopathological examination of the duodenal ulcer demonstrating the presence of up to 20 eosinophils per high-power field (HE 600x). D) Nails after corticosteroid therapy.
Discussion
The diagnosis of eosinophilic esophagitis requires characteristic symptoms of esophageal dysfunction such as vomiting, dysphagia, or feeding difficulties in the presence of at least 15 eosinophils/HPF on esophageal histology, as well as the absence of other possible causes for the eosinophilic infiltration such as gastroesophageal reflux disease, eosinophilic gastrointestinal disease, Crohn’s disease, among others [16]. Upon first clinical evaluation, EG was ruled out by means of a serial biopsy of the stomach and duodenum, which yielded normal results.
Duodenal ulcers usually have two main causes: Helicobacter pylori infection and use of NSAIDs [17]. Helicobacter pylori infection was excluded both by histological examination (excluding urease) and serology, which were negative. The patient was using PPI, which was promptly discontinued upon diagnosis of peptic ulcer, as the ulcer did not heal with the use of PPIs. At that time, a differential diagnosis of Crohn's disease was investigated, which was excluded. Crohn's disease can be the cause of duodenal ulcers, but it usually affects the distal small intestine and colon, and causes alterations in inflammatory tests. Duodenal involvement of Crohn’s disease may present ulcers, stenosis and fistula [18]. Due to the persistence of the active ulcer and symptoms, the endoscopy and biopsy of the duodenal ulcer were repeated, at which time the diagnosis of EG was reached.
We found reports of duodenal ulcers as a manifestation of EG in only twelve cases, which are summarized in Table 1. Due to the rarity of such cases, this diagnosis was not considered at first, especially due to the normal results of the duodenal mucosal biopsy.
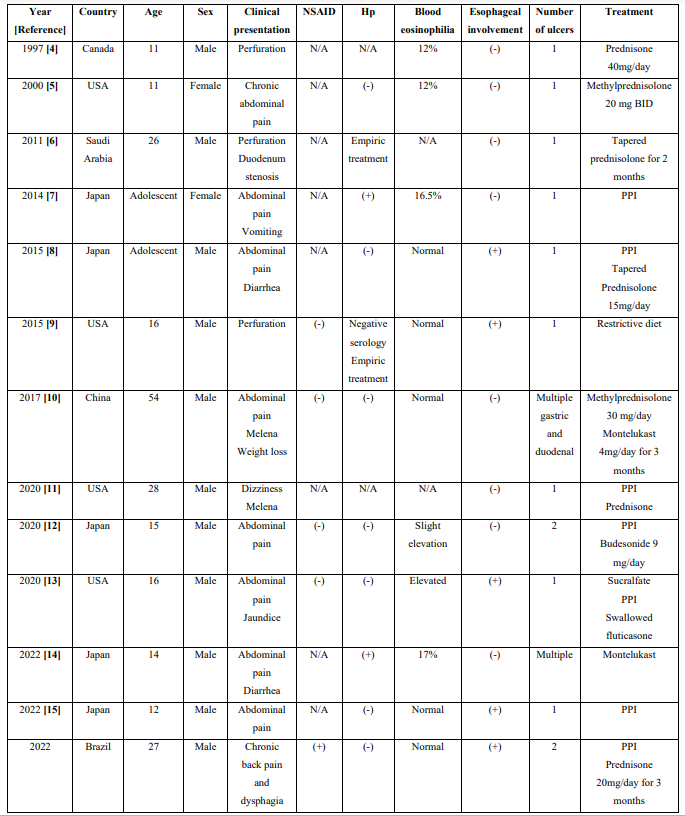
Table 1: Clinical characteristics of case reports of eosinophilic gastroenteritis-associated duodenal ulcers
NSAID: use of non-steroidal anti-inflammatory drugs; Hp: presence of Helicobacter pylori; (+): positive; (-) negative; USA: United States of America; N/A: not available; BID: twice a day, PPI: Proton Pump Inhibitor
With regard to food allergy, it is defined as a phenomenon in which adverse reactions are caused by allergen-specific immunological mechanisms following exposure to a given food. In some cases, certain fruits and vegetables show cross-reactivity with pollen such as birch pollen [19], which justifies the patient’s allergy tests findings.
It is important to emphasize that the patient's eosinophilic esophagitis did not improve with the combined diet, PPI, or swallowed fluticasone therapy. For patients who are refractory to first-line therapy, it is recommended to consider treatment with elemental diets, a combination of therapies, or clinical trials of new therapeutic agents [20]. In this clinical case, refractority was associated with EG.
In conclusion, we report a case where duodenal ulcers were a clinical manifestation of EG. We also aim to emphasize the importance of conducting a biopsy of ulcers that do not heal with PPI, considering that normal duodenal mucosal biopsy may not reveal eosinophilic infiltration.
References
- Leal RA, Narciso-Schiavon JL. (2014) Gastroenterite eosinofilica. Rev Soc Bras Clin Med. 12(3): 259-262. [Ref.]
- Chen PH, Anderson L, Zhang K, Weiss GA. (2021) Eosinophilic Gastritis/Gastroenteritis. Curr Gastroenterol Rep. 23(8): 1-13. [Ref.]
- Khan S. (2005) Eosinophilic gastroenteritis. Best Pract Res Clin Gastroenterol. 19(2): 177-198. [Ref.]
- Deslandres C, Russo P, Gould P, Hardy P. (1997) Perforated duodenal ulcer in a pediatric patient with eosinophilic gastroenteritis. Can J Gastroenterol. 11(3): 208-212. [Ref.]
- Markowitz JE, Russo P, Liacouras CA. (2000) Solitary duodenal ulcer: a new presentation of eosinophilic gastroenteritis. Gastrointest Endosc. 52(5): 673-676. [Ref.]
- Issa H, Bseiso B, Al-Salem AH. (2011) Eosinophilic enteritis presenting as a perforated duodenal ulcer. Endoscopy. 43 Suppl 2 UCTN: E358-359. [Ref.]
- Nakamura A, Iwaya Y, Iwaya M, Okamura T, Kobayashi S, et al. (2014) Eosinophilic gastroenteritis complicated with Helicobacter pylori infection unresponsive to eradication therapy. Intern Med. 53(18): 2061-2065. [Ref.]
- Yamazaki K, Sakashita T, Iwata H, Mizutani T, Matsuura K, et al. (2015) A case of a teenage boy with eosinophilic gastroenteritis with esophageal involvement developing a hemorrhagic duodenal ulcer. Clin J Gastroenterol. 8(4): 179-185. [Ref.]
- Riggle KM, Wahbeh G, Williams EM, Riehle KJ. (2015) Perforated duodenal ulcer: An unusual manifestation of allergic eosinophilic gastroenteritis. World J Gastroenterol. 21(44): 12709-12712. [Ref.]
- Chen B, Yang Z, Lu H, Wei C, Wang F, et al. (2017) Eosinophilic gastroenteritis presenting as upper gastrointestinal hematoma and ulcers after endoscopic biopsy: A case report and literature review. Medicine (Baltimore). 96(37): e8075. [Ref.]
- Priyadarshni S, Surapaneni BK, Dave K, Kaplan S, Patel N. (2020) Upper Gastrointestinal Bleed in a Young Male- A Rare Presentation of Eosinophilic Gastroenteritis. Cureus. 12(2): e7059. [Ref.]
- Kubo K, Kimura N, Mabe K, Matsuda S, Tsuda M, et al. (2020) Eosinophilic Gastroenteritis-associated Duodenal Ulcer Successfully Treated with Crushed Budesonide. Intern Med. 59(18): 2249-2254. [Ref.]
- Peck J, Kimsey KM, Harris E, Monforte H, Wilsey M. (2020) Solitary Duodenal Ulcer Causing Biliary Obstruction Requiring Rendezvous Procedure in a Pediatric Patient with Eosinophilic Gastroenteritis. Cureus. 12(7): e9377. [Ref.]
- Fujita Y, Tominaga K, Tanaka T, Ishida K, Yoshihara S. (2022) Eosinophilic Duodenal Ulcer Exacerbation after Helicobacter pylori Eradication in a 14-Year-Old Boy. Tohoku J Exp Med. 257(2): 153-156. [Ref.]
- Fujita Y, Tominaga K, Ishida K, Masuyama H, Yoshihara S. (2022) Proton Pump Inhibitor to Treat an Eosinophilic Duodenal Ulcer with Esophageal Involvement: A Pediatric Case. Tohoku J Exp Med. 257(4): 309-313. [Ref.]
- Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, et al. (2018) Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology. 155(4): 1022-1033.e10. [Ref.]
- Kamada T, Satoh K, Itoh T, Ito M, Iwamoto J, et al. (2021) Evidence-based clinical practice guidelines for peptic ulcer disease 2020. J Gastroenterol. 56(4): 303-322. [Ref.]
- Poggioli G, Stocchi L, Laureti S, Selleri S, Marra C, et al. (1997) Duodenal involvement of Crohn's disease: three different clinicopathologic patterns. Dis Colon Rectum. 40(2): 179-183. [Ref.]
- Kamei A, Izawa K, Ando T, Kaitani A, Yamamoto R, et al. (2022) Development of mouse model for oral allergy syndrome to identify IgE cross-reactive pollen and food allergens: ragweed pollen cross-reacts with fennel and black pepper. Front Immunol. 13: 945222. [Ref.]
- Strauss AL, Falk GW. (2022) Refractory eosinophilic esophagitis: what to do when the patient has not responded to proton pump inhibitors, steroids and diet. Curr Opin Gastroenterol. 38(4): 395-401. [Ref.]