>Corresponding Author : Nikolaos Andreas Chrysanthakopoulos
>Article Type : Research Article
>Volume : 3 | Issue : 1
>Received Date : 19 Dec, 2022
>Accepted Date : 29 Dec, 2022
>Published Date : 04 Jan, 2023
>DOI : https://doi.org/10.54289/JCRMH2300101
>Citation : Chrysanthakopoulos NA and Vryzaki E. (2023) Examination of the Possible Association Between Periodontal Indices and Risk of Renal Cell Carcinoma: A Case - Control Study. J Case Rep Med Hist 2(8): doi https://doi.org/10.54289/JCRMH2300101
>Copyright : © 2023 Chrysanthakopoulos NA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Research Article | Open Access
1Oncologist (MSc), Specialized in Clinical Oncology, Cytology and Histopathology, Dept of Pathological Anatomy, Medical School, University of Athens, Athens, Greece Resident in Maxillofacial and Oral Surgery, 401 General Military Hospital of Athens, Athens, Greece
2Department of Dermatology, Rio University Hospital of Patras, Greece, Running head: Periodontal Indices and Risk of Renal Cell Cancer
*Corresponding author: Nikolaos Andreas Chrysanthakopoulos, Oncologist (MSc), Specialized in Clinical Oncology, Cytology and Histopathology, Dept of Pathological Anatomy, Medical School, University of Athens, Athens, Greece Resident in Maxillofacial and Oral Surgery, 401 General Military Hospital of Athens, Athens, Greece
Abstract
Introduction: The relationship between Periodontal status and several types of cancer has been investigated over the past decades. The aim of the current research was to assess the effect of various risk factors on the risk of development of renal cell carcinoma in Greece using data form a population based case-control study design.
Material and Methods: Data from a population based case-control study and a clinical oral examination of 130 cases of renal cell carcinoma and 130 population controls were collected through a modified standardized questionnaire and a clinical examination and analyzed using univariate and logistic regression models. Periodontal condition contained the following indices, Probing Pocket Depth (PPD), Clinical Attachment Loss (CAL), Gingival Index (GI) and Bleeding on Probing (BOP).
Results: The presence of a renal cell cancer family history was associated with significantly increased adjusted OR [3,403-15,110]. Moreover, significantly increased adjusted ORs were found to be associated with cigarette smoking [1,433-5,049] and BOP [1,095-2,887], respectively.
Conclusions: The current research suggest positive associations with presence of renal cell cancer family history, smoking and presence of BOP in the etiology of renal cell cancer.
Keywords: Renal Cell Cancer; Periodontal Disease; Risk Factors; Adults
Abbreviations: PPD: Probing Pocket Depth, CAL: Clinical Attachment Loss, GI: Gingival Index, BOP: Bleeding on Probing, RCC: Renal cell cancer, ccRCC: Clear Cell Renal Cell Carcinoma, MS: Metabolic Syndrome, VDBP: Vitamin D-Binding Protein, PD: Periodontal Disease, ROS: Reactive Oxygen Species, RNI: Reactive Nitrogen Intermediates, IL: Interleukin, INF: Interferon, TNF: Tumor Necrosis Factor, CI: Confidence Interval, WHO: World Health Organization, BMI: Body Mass Index, VDBP: Vitamin D-Binding Protein, EMT: Epithelial-Mesenchymal
Introduction
Renal cell cancer (RCC) is responsible for around 3% of all malignancies in adults is the 12th most common cancer worldwide, and around 100,000 deaths recorded annually [1]. RCC represents 80-85% of renal cancers, is the most common renal variety, the third most commonly diagnosed urogenital malignancy [2] and is characterized by an increasing prevalence [3].
Subtypes of RCC include clear cell renal cell carcinoma (ccRCC), that is the most frequent histological type showing a prevalence of 75% of all primary renal cancers, whereas papillary and chromophobe RCC are two less common subtypes [4].
Despite the progress in diagnosis, a rate of 20-30% of all patients are diagnosed with metastatic disease. Metastatic RCC patients have a median survival of around 13 months, whereas the 5-year survival rate is less than 10% [5].
Renal cancers are more common in males than in females and the incidence has been increasing in advanced age, worldwide [1]. Demographic parameters, cigarette smoking, obesity, pathological conditions such as hypertension, hyperglycemia and hypertriglyceridemia belonging to the metabolic syndrome (MS) exposure to industrial or environmental agents, use of phenacetin containing analgetics, and lack of physical activity have been associated with RCC risk [6].
Factors that can also increase the risk of RCC include older age, as already has been mentioned, circulating vitamin D-binding protein (VDBP) concentration [7], treatment for renal failure, certain inherited syndromes, such as patients with von Hippel-Lindau disease, Birt-Hogg-Dube syndrome, tuberous sclerosis complex, hereditary papillary renal cell carcinoma or familial renal cancer, and family history of RCC [8].
Periodontal disease (PD), is a chronic inflammatory disease caused by bacterial infection which invades gingiva and periodontal supporting tissues [9], and is divided into two main types, gingivitis and periodontitis. In periodontitis periodontal bacteria [10] and viruses [11] are responsible for a host immuno-inflammatory response in periodontal tissues that leads to periodontal pocket formation, attachment loss and bone loss. PD prevalence and severity increases with age [12] and in case of aggressive and severe PD results in tooth loss [13]. Chronic PD risk factors are diabetes mellitus, and obesity [12].
Periodontal infection has systemic implications [14] and patients with PD show an increased risk of various pathological conditions such as ischemic heart disease [15], stroke [16], type 2 diabetes mellitus [14], osteoporosis [14], rheumatoid arthritis [17], respiratory diseases [18], and several types of human cancers [19]. An increased risk of total cancer [20,21] and certain location-specific types of cancer [22-24] have been associated with PD, tooth loss, and poor oral hygiene, independent of age, tobacco consumption, and alcohol consumption. At this time they remain unknown the exact mechanisms for the possible association between PD and risk for cancer development.
Periodontal pathogens could invade into the blood circulation and reach distant tissues [25] and to infect them via ingestion [26] or by aspiration into the lungs [27]. Those pathogens have been isolated in various organs such as lung aspirates [27], lymph nodes [28], arteries [29], precancerous gastric [30] and colon lesions [31], and esophageal [32] and colorectal cancers [33]. In those target locations periodontal bacteria may promote a permissive microenvironment favorable to cancer progression [31,34].
Chronic inflammation is considered as one of the main risk factors for cancer development. Periodontal infection is responsible for a chronic inflammation, and destruction of periodontal tissues [35], caused by elevated concentrations of circulating inflammatory biomarkers, whereas the spread of bacteria and inflammatory mediators can cause systemic inflammatory responses and damage to various organs [36]. Those responses induce cell proliferation and reactive oxygen species (ROS) reactive nitrogen intermediates (RNI) and other metabolites release that can promote cancer initiation [36,37].
Other crucial pro-inflammatory biomarkers that produced by periodontal bacteria such as Porphyromonas Gingivalis, Tannerella forsythia, Treponema denticola, Fusobacterium nucleatum, and Aggregatibacter actinomycetemcomitans [38], include the known cytokines and chemokines, such interleukin (IL)-1a, IL-1β, IL-6, IL-12, interferon-γ (INF-γ), and tumor necrosis factor (TNF)-α. Those biomarkers are able to induce oncogenic responses [39]. Poor oral health and periodontitis are associated with dysbiosis [40], that means alterations in the mouth bacterial communities, and in the immune status [41], which may be directly implicated in carcinogenesis. Very few research has investigated the association between PD indices or oral status and risk of kidney cancer development [21,42-44]. Supposing that a single epidemiological study may not be sufficient to determine the effect of PD on RCC risk, prospective and retrospective studies are required to further investigate and clarify the possible link between PD and RCC risk.
PD has been assessed by using self-report of periodontitis or using individual oral health assessments, such as measurements of PPD, CAL, and alveolar bone loss from radiographs, in observational studies. The wide variation in methods and criteria used for the periodontitis assessment and classification may explain in some extent the discrepancies observed across observational studies.
The current study is the first in Greece that investigated the possible association, as no previous epidemiological researches have been performed. The purpose of the current case-control study was to examine the possible association between PD indices and risk of RCC in a Greek adults sample.
Materials And Methods
Study population design and data collection
The current study was carried out between June 2021 and April 2022 and consisted of 260 participants who recruited from two private medical and a dental practice. The study sample size was calculated by the EPITOOLS guidelines (https://epitools.ausvet.com.au) determined with 95% Confidence Interval (CI) and desired power 0.8. The World Health Organization (WHO) recommendations [45] for assessing periodontal status incidence were used for assessing age group.
The study sample consisted of 260 individuals, 130 who suffered from RCC - cases and 130 healthy individuals - control, aged 48 to 77.
The diagnosis of RCC made by the same physicians and derived from their medical files. Most cases of RCC are strongly suspected and diagnosed by US and further investigated by CT scan, whereas MRI may provide additional data. However the definitive diagnosis based on a renal tumor core biopsy as provides histopathological confirmation of malignancy with high specificity and sensitivity, and before the application of any treatment such as surgery, chemotherapy, radiation therapy, targeted therapy, and immunotherapy [46].
Eligibility Criteria
To be eligible, the participants, cases and controls, should not have been treated by a conservative or a surgical process in their oral cavity in the last 6 months, or prescribed for systemic antibiotic regimens or immunosuppression agents or glucocorticoids within the previous 6 months. They should also have more than 15 teeth and periodontitis from stage I to IV [47].
Potential participants were excluded if they had cardiovascular diseases, diabetes mellitus, or any type of other types of cancer. The mentioned diseases could affect oral and periodontal tissues and result in biased secondary associations.
Individuals derived from the friendly and collegial environment of cases, were resident of the same city with cases, and were presented to routine health follow-up at the mentioned practices determined the controls group. Moreover, cases and controls were matched for gender and age, whereas advanced RCC patients under medical treatment/metastatic disease, and hospital patients were excluded from the study protocol.
The current study as a non-experimental one was not approved by authorized committees (Ministry of Health, etc.). All participants were informed about the aims/methods and significance of the current research, and gave their written consent to enroll in the study protocol.
Research Questionnaire
A modified Minnesota Dental School Medical Questionnaire [48], was given to all participants. Participants were asked to report their age, gender, smoking status, socio-economic and educational status, and data derived from their past Dental / Medical history.
For assessing the intra-examiner variance a randomly selected sample of 60 (20%) individuals re-examined clinically after a period of 21 days, without giving oral hygiene instructions to the participants, by the same Dentist.
No differences were recorded between the 1st and the 2nd clinical examination (Cohen's Kappa = 0.97).
Assessment of Periodontal Disease indices
All interproximal sites, mesial and distal, were measured using the following indices, PPD, CAL, the gingival condition (Gingival Index, GI) and BOP in all quadrants apart from remaining roots and third molars. The worst values of the indices assessed to the nearest1.0 mm and coded as dichotomous variables for each individual, using a Williams probe with a controlled force of 0.2 N (DB764R, Aesculap AG & Co. KG, Tuttlingen, Germany). PPD was dichotomously assessed as score 0: stage I [maximum PPD ≤ 4.0 mm] and score 1: stage II-IV [PPD ≤ 4.0 - ≥ 6.0 mm], and CAL severity was assessed as score 0: stage I [CAL: 1.0-2.0 mm], and score 1: stage II-IV [CAL: 3.00 - ≥ 5.0 mm] [47].
GI classified as score 0, which corresponds to Löe [49] classification 0 and 1, and-score 1,which corresponds to Löe classification as score 2 and 3. BOP was coded as score 0: absence, and score 1: presence of BOP and regarded positive if it occurred within 15 seconds of probing [50].
Assessment of covariates
Socio-demographic variables, and potential variables regarded as indicators or risk factors for RCC included as covariates in the univariate and multivariable analyses. Education status was classified as elementary level and graduated from University/College. Socio-economic status was classified as ≤ 1,000 and >1,000 €/month. Cigarette smoking was categorized as never (individuals who smoked <100 cigarettes during their lifetime), and former (individuals who smoked at least 100 cigarettes in their lifetime and reported that they now smoke “not at all”)/current smokers (individuals who smoked at least 100 cigarettes in their lifetime and reported they now smoke “every day” or “some days”). Body Mass Index (BMI) is an obesity index and was classified as normal (<30 Kg/m2) and high (≥30 Kg/m2) and is considered as a risk factor for RCC development [51]. Hypertension is present if the resting blood pressure is insistently ≥ 130/80 or 140/90 mmHg [52]. Phenacetin-based analgesics have been linked to the development of RCC, based on received doses per week as regular (≤ 2 times a week for 1 month or longer, <2.0 gr), and maximum (dose >2 gr/weekly). [53] Exposure to environmental/ industrial agents included workers in plastic, petroleum/gasoline, coal, mineral, and oil, industries. Familial history cancer concerned RCC in first-degree relatives [54].
Statistical Analysis
The worst values of PD indices for cases and controls were coded as 1.
Females, never smokers, low socioeconomic [income/monthly ≤ 1,000 €] and educational level [graduated from Elementary/High School], normal BMI, absence of previous kidney failure treatment, RCC family history, previous exposure to industrial/ environmental agents, previous phenacetin usage hypertension, and controls were coded as 0. Cases and controls age distribution was coded as 0, 1, 2, and 3 for ages 48-50, 51-60, 61-70 and 71+, respectively.
Univariate analysis (chi-square) model was applied to assess the associations between the independent indices examined and cases/controls, separately. Multivariate logistic regression analysis model was performed to estimate the associations between the dependent variable, RCC, and independent ones using the Enter and Stepwise methods. Unadjusted and Adjusted Odds Ratios [OR's] and 95% [Confidence Interval] CI were also estimated. The statistical model Cohran’s and Mantel-Haenszel’s was applied to control possible confounders, such as smoking, SES and educational status.
The SPSS statistical package [SPSS PC20.0, SPSS, Inc., Chicago, IL, USA], was performed for statistical analysis, and a p value less than 5% [p< 0.05] was considered to be statistically significant.
Results
The mean age of cases and controls was 56.6 ± 3.5 and 58.3 ± 4.7 years, respectively. The main histological type was clear cell renal cell carcinoma (ccRCC), and in the study protocol were not included the other types of RCC as its etiology differs. Table 1 presents the results after performing the univariate analysis model. The model indicated that previous/current smokers [p = 0.04, 95% CI = 0.472 (0.281-0.793)], family history [ p=0.000, 95% CI = 0.206 (0.107-0.399)], and BOP [p = 0.009, 95% CI = 0.506 (0.302-0.849)], were statistically significantly associated with risk for RCC development. Table 1 also shows Unadjusted Odds Ratio and 95% CI for each parameter examined.
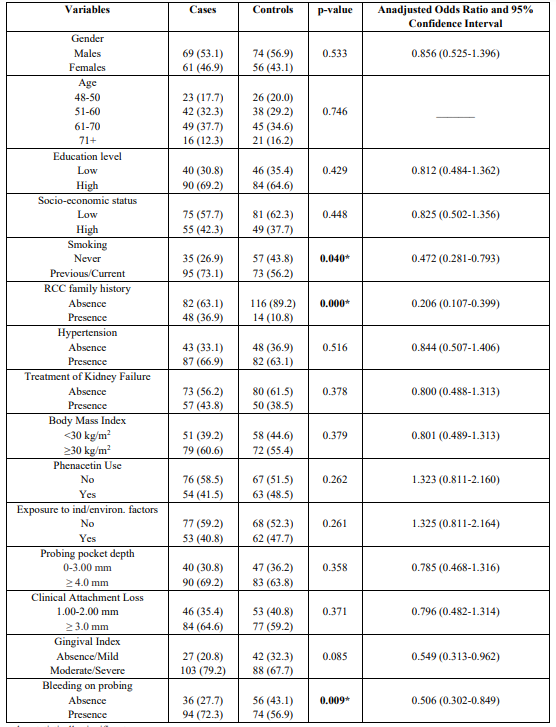
Table 1: Univariate analysis of cases and controls regarding each independent variable
* p-value statistically significant
After performing the 1st model (step 1a) of the logistic regression analysis the same indices were found to be statistically significantly associated with RRC risk. Adjusted Odds Ratio and 95% CI for each variable examined is presented in the Table 2.
Similarly, the final model (step 12a) of multivariate regression analysis is presented in Table 2 and showed that previous/current smokers [p = 0.02, 95% CI = 1,433-5,049], family history [p = 0.000, 95% CI = 3,403-15,110], and BOP [p = 0.048, 95% CI = 1,095-2,887] were statistically significantly associated with risk for RCC development.
Tables 3 indicates the outcomes after applying the statistical model Cohran’s and Mantel-Haenszel’s for controlling possible confounders, such as gender, smoking, and SES. It is obvious that smoking status is not a confounder.
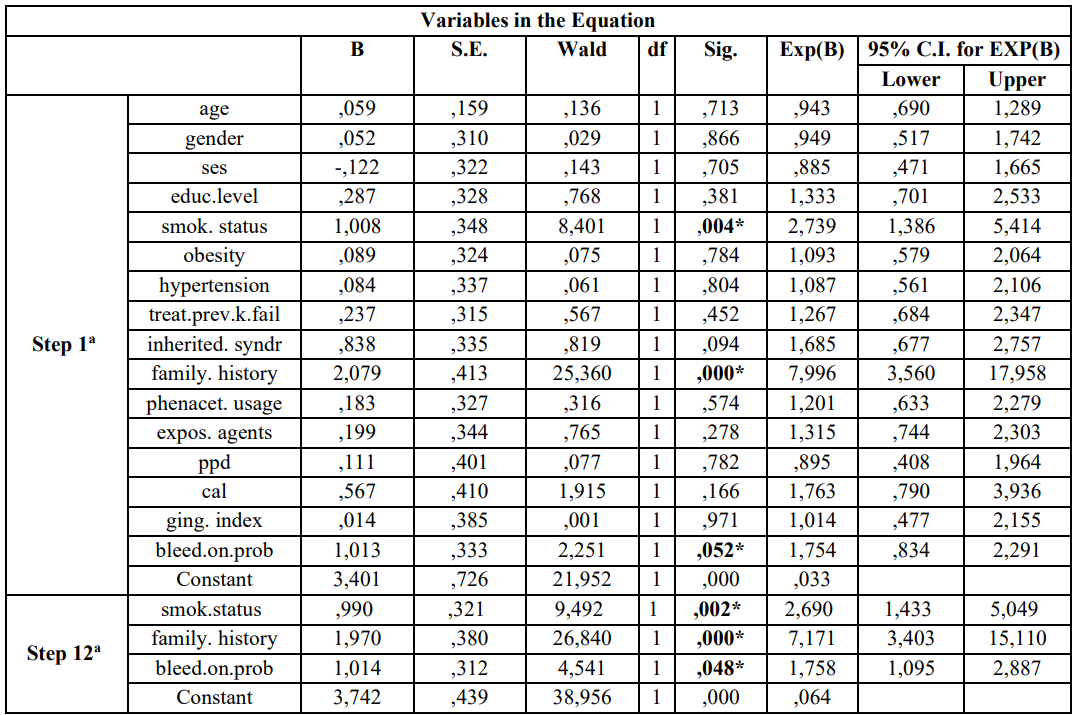
Table 2: Multivariate logistic regression analysis model of cases and controls regarding risk factors examined and RCC according to Enter (first step-1a) and Wald (last step 12a)
a. Variable(s) entered on step 1: age, gender, ses, educ.level, smok.status, obesity, hypertension, treat.prev.k.fail, family. History, phenacet. Usage, expos. agents, ppd, cal, ging.index, bleed.on.prob.
* p-value statistically significant
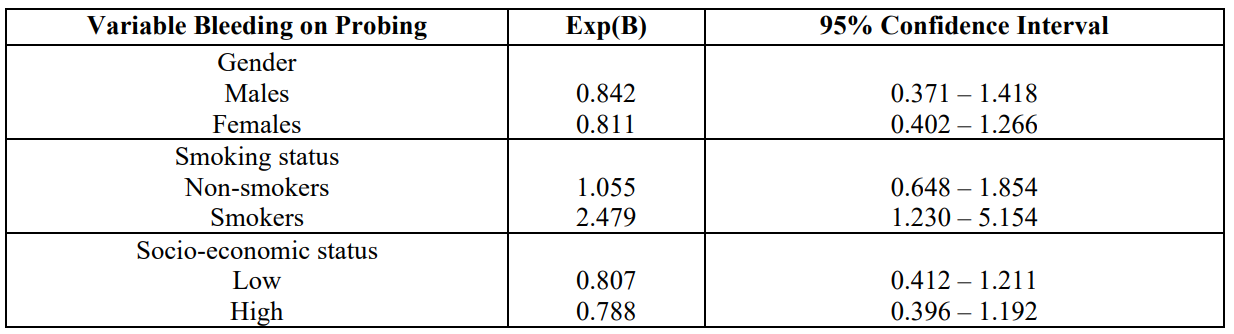
Table 3: Cohran’s and Mantel-Haenszel’s statistical method for controlling known Confounders
Discussion
The outcomes revealed that individuals with a RCC family history, previous/current smokers, and BOP were at significantly higher risk for developing RCC.
The exact etiology of RCC remains unclear and various modifiable and unmodifiable risk factors have been proposed as contributing factors, such as male gender, advanced age [1], cigarette smoking, obesity, hypertension, hyperglycemia and hypertriglyceridemia, exposure to industrial/ environmental agents, use of phenacetin containing analgetics, and lack of physical activity [6].
Additional risk factors include [7] circulating vitamin D-binding protein (VDBP) concentration, treatment for renal failure, certain inherited syndromes, such as patients with von Hippel-Lindau disease, Birt-Hogg-Dube syndrome, tuberous sclerosis complex, hereditary papillary RCC or familial renal cancer, and family history of RCC [8].
No associations between advanced age, male gender, hypertension, treatment for renal failure, increased BMI, previous exposure to industrial/environmental agents and use of phenacetin containing analgetics were recorded.
RCC risk found to be associated with cigarette smoking, finding that was in accordance with the results of previous researches and is considered as an established risk factor for RCC [55,56].
Moreover, both current and former smokers have an increased risk of developing RCC, with heavy smokers showing the highest incidence [55,57].
Smoking seems to induce renal damage by several mechanisms, including tubulotoxic effects, hemodynamic changes, endothelial cell dysfunction, and oxidative stress [58], which are potentially implicated in carcinogenesis and cancer progression. Tobacco smoking is responsible for genetic and epigenetic abnormalities such as gene mutations, deletions, and DNA methylation.
Polycyclic aromatic hydrocarbons in tobacco smoke can induce mutations in the p53 gene, which can potentially result in cancer progression [59]. Smoking has been suggested to have an effect on the immune system, promoting inflammation and suppressing the immune function by reducing T-cell and natural killer cell activation. Inflammation and immune suppression could result in neoplastic growth [60].
Similarly, a previous family RCC history was statistically significantly associated with RCC risk. Previous researches have confirmed such a finding [61,62]. Similar reports have reported associations ranging from none to a five-fold increase in risk [63-66]. One of those indicated that almost 60% of RCC patients had a first- or second-degree relative with RCC [64], suggesting a strong genetic component to risk. On the contrary, a Canadian case-control study did not identify any association between family history and RCC [67], and two similar reports recorded a nonsignificant increased risk for individuals with at least one first degree relative with RCC [68,69]. RCC cases occur sporadically, however, several hereditary conditions, such as VHL syndrome [67], hereditary papillary renal cancer related to germline mutations, activation of the MET and the FH gene [70], and BHD [71], have been linked to RCC development. However, the mentioned syndromes are rare and possibly most of the familial risk is not attributed to these highly penetrant genes [72]. Other lower penetrance genes may exist with higher frequency in the population and may are responsible for more cases of RCC [73]. Previous surveys revealed higher risks among siblings than among parents and offspring [64,65,68].
Chronic inflammation can result in carcinogenesis [74], and the pathway of cancer-related inflammation is the recruitment of leukocytes, production of cytokines and chemokines, and subsequent progression, angiogenesis, epithelial-mesenchymal transition (EMT), migration, and metastasis [75]. Activated inflammatory cells, neutrophils, macrophages, and dendritic cells, secrete pro-inflammatory and pro-growth cytokines and chemokines, such as matrix metalloproteases, pro-angiogenic molecules, TNF-α, ROS and RNI species that can induce DNA damage in epithelial cells and produce an environment for both initiation and progression of carcinogenesis at local and distant locations [76].
PD and periodontitis as a chronic inflammation is responsible for releasing of bacterial and inflammatory mediators into saliva and, to a lesser degree, into blood circulation. Periodontal pathogens might promote cancer development through invasion of blood vessels, bacteremia, and subclinical infection in distant locations [77]. Those pathogens also have essential roles in the carcinogenesis of distant organs, such as Porphyromonas gingivalis and Fusobacterium nucleatum [26,78]. Porphyromonas gingivalis could penetrate and invade various epithelial cells, affecting the cell cycle and preventing epithelial cell apoptosis, a landmark of cancer development [79].
Oral bacteria metabolism carcinogenic by-products are suggested to be important in the link between PD and cancer. PD may influence carcinogenesis through the increased production of carcinogens, mainly nitrosamines. Individuals with PD and poor oral hygiene have considerably increased levels of oral bacteria and nitrosamine levels in their oral cavity due to the presence of nitrate-reducing bacteria [80]. Poor oral hygiene and loss of teeth might lead to greater endogenous nitrosamine production and consequently a greater risk of cancer.
However, it remains unclear whether PD directly increases cancer risk or shared genetic and/or environmental etiological factors are also involved. Dysbiosis of the oral microbiota, bacteria induced immune evasion and dysregulation, activation of various cellular signaling pathways, and subsequent inhibition of apoptosis and activation of cell proliferation in patients with chronic PD have been suggested as pro-tumorigenic mechanisms [81]. Some of the genes consistently associated with aggressive periodontitis, such as COX2 and CDKN2B are also associated with cancer, observation that suggests shared genetic susceptibility between PD and cancer [34].
BOP is a critical periodontal diagnosis indicator, the most reliable PD activity indicator [82], and an indicator of disease progression [83], as can be effective in the diagnosis and monitoring of active PD [84,85] and sites that BOP tend to have significantly more inflammation than nonbleeding sites. As a PD index, BOP indicates the host’s vascular response in terms of hyperemia, the dilation of capillaries and increased blood flow in the inflammation location. BOP is also a widely used criterion to diagnose gingival inflammation, however it has been suggested that periodontal pockets with a probing depth of ≥5.00 mm showed a significantly higher incidence of BOP [82]. In most cases, BOP is an earlier inflammation sign than gingival color changes [86]. Depending on the inflammation severity bleeding can vary from a thin red line along the gingival sulcus to abundant bleeding [83].
The etiological relationship between PD and RCC remains unknown, and little is known about their underlying mechanisms. Few previous studies have investigated the mentioned association. Hujoel et al. [42] recorded that gingival inflammation could be a risk factor for several types of cancer development. A similar article showed that periodontitis was associated with a 33% increase in risk for smoking-related RCC [43]. Significant associations for individuals with a history of PD were revealed for kidney cancer and after adjusting for known risk factors, including detailed smoking history and dietary factors (HR = 1.49, 95% CI = 1.12-1.97). However, no association was found when limited to never‐smokers alone (HR = 1.06, 95%CI = 0.61‐1.85) [21].
In another report among postmenopausal females no significant association was observed between PD indices and risk of kidney cancer [HR = 1.09 95% CI = 0.76-1.56] [44].
It could be hypothesized that deleterious agents derived from smoking metabolism and biomarkers derived from the chronic inflammation of periodontal tissues are responsible for RCC development.
Strengths of the current research concern the completeness of follow-up, the control of possible confounding and interaction by known risk factors. The assessment of PD status by oral clinical examination and not by a self-report questionnaire, led to an unbiased classification of exposure to PD. Self-reported data could result in misclassification and thus to the underestimation of the association examined.
Residual confounding by smoking may be a major limitation as it is a known confounder. After adjusting for common confounders, including, gender, smoking, and SES we cannot exclude residual confounding by smoking to explain the recorded association. It is obvious that further research is required to investigate the possible association between periodontal status and risk of RCC development.
Conclusion
In conclusion, individuals with a RCC cancer family history, smoking, and BOP were at significantly higher risk for developping RCC, after adjusting for known confounders.
References
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, et al. (2015) GLOBOCAN 2012, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No.11Lyon. France: International Agency for Research on Cancer 2013. [Ref.]
- Landis SH, Murray T, Bolden S, Wingo PA. (1999) Cancer statistics, 1999. CA Cancer J Clin. 49(1): 8-31. [PubMed.]
- Ljunberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, et al. (2011) The epidemiology of renal cell carcinoma. Eur Urol. 60: 615-621. [PubMed.]
- Aydin H, Chen L, Cheng L, Vaziri S, He H, et al. (2010) Clear cell tubulopapillary renal cell carcinoma: a study of 36 distinctive low-grade epithelial tumors of the kidney. Am J Surg Pathol. 34(11): 1608-1621. [PubMed.]
- Cairns P. (2010) Renal cell carcinoma. Cancer Biomark. 9(1-6): 461-473. [PubMed.]
- Chow WH, Dong LM, Devesa SS. (2010) Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 7(5): 245-257. [PubMed.]
- Mondul AM, Weinstein SJ, Moy KA, Männistö S, Albanes D. (2014) Vitamin D-binding protein, circulating vitamin D and risk of renal cell carcinoma. Int J Cancer. 134(11): 2699-2706. [PubMed.]
- Zbar B, Tory K, Merino M, Schmidt L, Glenn G, et al. (1994) Hereditary papillary renal cell carcinoma. J Urol. 151: 561. [PubMed.]
- Highfeld J. (2009) Diagnosis and classification of periodontal disease. Aust Dent J. 54: S11-26. [PubMed.]
- Loesche WJ, Grossman NS. (2001) Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clin Microbiol Rev. 14(4): 727-572. [PubMed.]
- Grinde B, Olsen I. (2010) The role of viruses in oral disease. J Oral Microbiol. 12: 2. [PubMed.]
- Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, et al. (2015) Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J Periodontol. 86: 611-622. [PubMed.]
- Kinane DF, Stathopoulou PG, Papapanou PN. (2017) Periodontal diseases. Nat Rev Dis Primers. 3: 17038. [PubMed.]
- Kim J, Amar S. (2006) Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 94: 10-21. [PubMed.]
- Genco R, Offenbacher S, Beck J. (2002) Periodontal disease and cardiovascular disease: epidemiology and possible mechanisms. J Am DentAssoc. 133 Suppl: 14S-22S. [PubMed.]
- Joshipura KJ, Hung HC, Rimm EB, Willett WC, Ascherio A. (2003) Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. 34: 47-52. [PubMed.]
- Ortiz P, Bissada N, Palomo L, Han YW, Al-Zahrani MS, et al. (2009) Periodontal therapy reduces the severity of active rheumatoid arthritis in patients treated with or without tumor necrosis factor inhibitors. J Periodontol. 80: 535-540. [PubMed.]
- Scannapieco FA, Bush RB, Paju S. (2003) Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic review. Ann Periodontol. 8: 54-69. [PubMed.]
- Fitzpatrick SG, Katz J. (2010) The association between periodontal disease and cancer: a review of the literature. J Dent. 38: 83-95. [PubMed.]
- Wen BW, Tsai CS, Lin CL, Chang YJ, Lee CF, et al. (2014) Cancer risk among gingivitis and periodontitis patients: a nationwide cohort study. QJM. 107: 283-290. [PubMed.]
- Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. (2008) Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 9: 550-558. [PubMed.]
- Tezal M, Sullivan MA, Hyland A, Marshall JR, Stoler D, et al. (2009) Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Bio-markers Prev. 18: 2406-2412. [PubMed.]
- Abnet CC, Qiao YL, Mark SD, Dong ZW, Taylor PR, et al. (2001) Prospective study of tooth loss and incident esophageal and gastric cancers in China. Canc Caus Control. 12: 847-854. [PubMed.]
- Stolzenberg-Solomon RZ, Dodd KW, Blaser MJ, Virtamo J, Taylor PR, et al. (2003) Tooth loss, pancreatic cancer, and Helicobacter pylori. Am J Clin Nutr. 78: 176-181. [PubMed.]
- Loos BG. (2005) Systemic markers of inflammation in periodontitis. J Periodontol. 76: 2106-2115. [PubMed.]
- Gao S, Li S, Ma Z, Liang S, Shan T, et al. (2016) Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect. Agent Cancer. 11: 3. [PubMed.]
- El-Solh AA, Pietrantoni C, Bhat A, Aquilina AT, Okada M, et al. (2003) Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 167: 1650-1654. [PubMed.]
- Rajakaruna GA, Umeda M, Uchida K, Furukawa A, Yuan B, et al. (2012) Possible translocation of periodontal pathogens into the lymph nodes draining the oral cavity. J Microbiol. 50: 827-836. [PubMed.]
- Gaetti-Jardim E Jr, Marcelino SL, Feitosa AC, Romito GA, Avila-Campos MJ. (2009) Quantitative detection of periodontopathic bacteria in atherosclerotic plaques from coronary arteries. J Med Microbiol. 58: 1568-1575. [PubMed.]
- Salazar CR, Sun J, Li Y, Francios F, Corby P, et al. (2013) Association between selected oral pathogens and gastric precancerous lesions. PLoS One. 8: e51604. [Ref.]
- Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, et al. (2013) Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 14: 207-215. [PubMed.]
- Narikiyo M, Tanabe C, Yamada Y, Igaki H, Tachimori Y, et al. (2004) Frequent and preferential infection of Treponema denticola, Streptococcus mitis, and Streptococcus anginosus in esophageal cancers. Cancer Sci. 95: 569-574. [Ref.]
- Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, et al. (2012) Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 22: 299-306. [PubMed.]
- Perera M, Al-Hebshi NN, Speicher DJ, Perera I, Johnson NW. (2016) Emerging role of bacteria in oral carcinogenesis: a review with special reference to periopathogenic bacteria. J Oral Microbiol. 8: 10. [Ref.]
- Wang GP. (2015) Defining functional signatures of dysbiosis in periodontitis progression. Genome Med.7(1): 40. [Ref.]
- Corbella S, Veronesi P, Galimberti V, Weinstein R, Fabbro MD et al. (2018) Is periodontitis a risk indicator for cancer? A meta-analysis. PloS One. 13(4): e0195683. [Ref.]
- Hoare A, Soto C, Rojas-Celis V, Bravo D. (2019) Chronic inflammation as a link between periodontitis and carcinogenesis. Mediat Inflamm. 2019: 1029857. [PubMed.]
- Michaud DS, Izard J, Wilhelm-Benartzi CS, You DH, Grote VA, et al. (2013) Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut. 62: 1764-1770. [PubMed.]
- Meurman JH. (2010) Oral microbiota and cancer. J Oral Microbiol. 2010: 2. [PubMed.]
- Hajishengallis G, Lamont RJ. (2012) Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 27: 409-419. [PubMed.]
- Hajishengallis G, Sahingur SE. (2014) Novel inflammatory pathways in periodontitis. Adv Dent Res. 26: 23-29. [Ref.]
- Hujoel PP, Drangsholt M, Spiekerman C, Weiss NS. (2003) An exploration of the periodontitis-cancer association. Ann Epidemiol. 13: 312-316. [PubMed.]
- Michaud DS, Kelsey KT, Papathanasiou E, Genco CA, Giovannucci E. (2016) Periodontal disease and risk of all cancers among male never smokers: an updated analysis of the Health Professionals Follow-up Study. Ann Oncol. 27: 941-947. [PubMed.]
- Nwizu NN, Marshall JR, Moysich K, Genco RJ, Hovey KM, et al. (2017) Periodontal Disease and Incident Cancer Risk among Postmenopausal Women: Results from the Women’s Health Initiative (WHI) Observational Cohort. Cancer Epidemiol Biomarkers Prev. 26(8): 1255-1265. [PubMed.]
- World Health Organization. (1997) Oral health surveys: basic methods. Geneva: World Health Organization. [Ref.]
- Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, et al. (2019) Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 30: 706-720. [PubMed.]
- Tonetti MS, Greenwell H, Kornman KS. (2018) Staging and grading of periodontitis: framework and proposal ofa new classification and case definition. J Clin Periodontol 45: S149-S161. [PubMed.]
- Molloy J, Wolff LF, Lopez-Guzman A, Hodges JS. (2004) The association of periodontal disease parameters with systemic medical conditions and tobacco use. J Clin Periodontol. 31: 625-632. [PubMed.]
- Löe H. (1967) The Gingival Index, the Plaque Index, and the Retention Index Systems. J Periodontol. 38(6): 610-616. [PubMed.]
- Peikert SA, Mittelhamm F, Frisch E, Vach K, Ratka Krüger P, et al. (2020) Use of digital periodontal data to compare periodontal treatment outcomes in a practice-based research network (PBRN): a proof of concept. BMC Oral Health. 20: 297. [Ref.]
- Aurilio G, Piva F, Santoni M, Cimadamore A, Sorgentoni G, et al. (2019) The Role of Obesityin Renal Cell Carcinoma Patients: Clinical-Pathological Implications. Int J Mol Sci. 20(22): 5683. [Ref.]
- Poulter NR, Prabhakaran D, Caulfield M. (2015) Hypertension. Lancet. 386 (9995): 801-812. [PubMed.]
- Gago-Dominguez M, Yuan JM, Castelao JE, Ross RK, Yu MC. (1999) Regular use of analgesics is a risk factor for renal cell carcinoma. Brit J Cancer. 81(3): 542-548. [PubMed.]
- Clague J, Lin J, Cassidy A, Matin S, Tannir NM, et al. (2009) Family history and risk of Renal Cell Carcinoma: results from a case-control study and systematic meta-analysis. Cancer Epidemiol Biomarkers Prev. 18(3): 801-807. [PubMed.]
- Hunt JD, van der Hel OL, McMillan GP, Boffetta P, Brennan P. (2005) Renal cell carcinoma in relation to cigarette smoking: Meta-analysis of 24 studies. Int J Cancer. 114: 101-108. [PubMed.]
- Flaherty KT, Fuchs CS, Colditz GA, Stampfer MJ, Speizer FE, et al. (2005) A prospective study of body mass index, hypertension, and smoking and the risk of renal cell carcinoma (United States). Cancer Causes Control. 16: 1099-1106. [PubMed.]
- Theis RP, Grieb SMD, Burr D, Siddiqui TT, Asal NR. (2008) Smoking, environmental tobacco smoke, and risk of renal cell cancer: A population-based case-control study. BMC Cancer. 8: 387. [Ref.]
- Orth S. (2002) Cigarette smoking: An important renal risk factor-Far beyond carcinogenesis. Tob Induc Dis. 1: 137-155. [PubMed.]
- Kudahetti S, Fisher G, Ambroisine L, Foster C, Reuter V, et al. (2009) Immunochemistry is an independent prognostic marker for outcome in conservatively treated prostate cancer. BJU Int. 104: 20-24. [PubMed.]
- Mehta H, Nazzal K, Sadikot RT. (2008) Cigarette smoking and innate immunity. Inflamm Res. 57: 497-503. [PubMed.]
- Cohen AJ, Li FP, Berg S, Marchetto DJ, Tsai S, et al. (1979) Hereditary renal-cell carcinoma associated with chromosomal translocation. N Engl J Med. 301: 592-595. [PubMed.]
- Franksson C, Bergstrand A, Ljungdahl I, Magnusson G, Nordenstam H. (1972) Renal carcinoma (hypernephroma) occurring in 5 siblings. J Urol. 108: 58-61. [Ref.]
- Gago-Dominguez M, Yuan JM, Castelao JE, Ross RK, Yu MC. (2001) Family history and risk of renal cell carcinoma. Cancer Epidemiol Biomarkers Prev. 10: 1001-1004. [PubMed.]
- Gudbjartsson T, Jonasdottir TJ, Thoroddsen A, Einarsson GV, Jónsdóttir GM, et al. (2002) A population-based familial aggregation analysis indicates genetic contribution in a majority of renal cell carcinomas. Int J Cancer. 100: 476-479. [PubMed.]
- Negri E, Foschi R, Talamini R, Montella M, Ramazzotti V, et al. (2006) Family history of cancer and the risk of renal cell cancer. Cancer Epidemiol Biomarkers Prev. 15: 2441-2444. [PubMed.]
- Kreiger N, Marrett LD, Dodds L, Hilditch S, Darlington GA. (1993) Risk factors for renal cell carcinoma: results of a population-based case-control study. Canc Caus Control. 4: 101-110. [PubMed.]
- Latif F, Tory K, Gnarra J, Yao M, Duh FM, et al. (1993) Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 260: 1317-1320. [PubMed.]
- Hung RJ, Moore L, Boffetta P, Feng BJ, Toro JR, et al. (2007) Family history and the risk of kidney cancer: a multicenter case control study in Central Europe. Cancer Epidemiol Biomarkers Prev. 16: 1287-1290. [PubMed.]
- Randi G, Pelucchi C, Negri E, Talamini R, Galeone C, et al. (2007) Family history of urogenital cancers in patients with bladder, renal cell and prostate cancers. Int J Cancer. 121: 2748-2752. [PubMed.]
- Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EEM, et al. (2002) Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 30: 406-410. [PubMed.]
- Nickerson ML, Warren MB, Toro JR, Matrosova V, Glenn G, et al. (2002) Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell. 2: 157-164. [PubMed.]
- Peto J, Houlston RS. (2001) Genetics and the common cancers. Eur J Cancer. 37: S88-S96. [PubMed.]
- Hemminki K, Li X. (2004) Familial renal cell cancer appears to have a recessive component. J Med Genet. 41: e58. [Ref.]
- Rakoff-Nahoum S. (2006) Why cancer and inflammation? Yale J Biol Med. 79: 123-130. [PubMed.]
- Hughes CE, Nibbs RJB. (2018) A guide to chemokines and their receptors. FEBS J. 285 (16): 2944-2971. [Ref.]
- Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. (2007) Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 13(Suppl 4): 3-10. [PubMed.]
- Dizdar O, Hayran M, Guven DC, Yılmaz TB, Taheri S, et al. (2017) Increased cancer risk in patients with periodontitis. Curr Med Res Opin. 33: 2195-2200. [PubMed.]
- Mima K, Cao Y, Chan AT, Qian ZR, Nowak JA, et al. (2016) Fusobacterium nucleatum in colorectal carcinoma tissue according to tumor location. Clin Translational Gastroenterol. 27 (11): e200. [PubMed.]
- Katz J, Onate MD, Pauley KM, Bhattacharyya I, Cha S. (2011) Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int J Oral Sci. 3: 209-215. [PubMed.]
- Nair J, Ohshima H, Nair UJ, Bartsch H. (1996) Endogenous formation of nitrosamines and oxidative DNA-damaging agents in tobacco users. Crit Rev Toxicol. 26: 149-161. [PubMed.]
- Arora M, Weuve J, Fall K, Pedersen NL, Mucci LA. (2010) An exploration of shared genetic risk factors between periodontal disease and cancers: a prospective co-twin study. Am J Epidemiol. 171(2): 253-259. [PubMed.]
- Lang NP, Joss A, Orsanic T, Gusberti FA, Siegrist BE. (1986) Bleeding on probing. A predictor for the progression of periodontal disease? J Clin Periodontol. 13(6): 590-596. [PubMed.]
- Newbrun E. (1996) Indices to measure gingival bleeding. J Periodontol. 67: 555-561. [PubMed.]
- Haffajee AD, Socransky SS, Lindhe J, Kent RL, Okamoto H, et al. (1991) Clinical risk indicators for periodontal attachment loss. J Clin Periodontol. 18: 117-125. [PubMed.]
- Greenstein G, Caton J, Polson AM. (1981) Histologic characteristics associated with bleeding after probing and visual signs of inflammation. J Periodontol. 52: 420-425. [PubMed.]
- Newman MG. (2019) Periodontal Examination and Diagnosis: in Newman and Carranza's Clinical Periodontology, Elsevie. 32: 378-396.e3. [PubMed.]