>Corresponding Author : Javad S. Fadardi
>Article Type : Case Report
>Volume : 3 | Issue : 3
>Received Date : 12 March, 2023
>Accepted Date : 24 March, 2023
>Published Date : 31 March, 2023
>DOI : https://doi.org/10.54289/JCRMH2300113
>Citation : Khorrami Z, Fadardi JS, Rahimi MD and Bastami M. (2023) Outcomes of a Cognitive Management Program (CMP) for an Individual with Mild Neurocognitive Disorder at Risk of Developing Alzheimer. J Case Rep Med Hist 3(3): doi https://doi.org/10.54289/JCRMH2300113
>Copyright : © 2023 Khorrami Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access
1Ferdowsi University of Mashhad, Iran
2Bangor University, UK
3Claremont Graduate University, USA
4University of Tehran, Iran
*Corresponding author: Dr. Javad Salehi Fadardi, School of Education and Psychology, Ferdowsi University of Mashhad, Iran; e-mail: J.s.fadardi@um.ac.ir; phone: +985138805867
Abstract
Individuals with mild neurocognitive disorder (mild-NCD) may suffer from one or a range of mild neurocognitive dysfunctions that disturb their daily life routines. This study tested the outcomes of an author-compiled cognitive management program (CMP) on a patient at the risk of developing Alzheimer. The CMP comprised a careful diagnostic procedure, a multi-dimensional cognitive assessment of the participant's cognitive functioning, and a series of cognitive rehabilitation procedures. A 59-year old woman (diagnosed based on DSM-5 and Addenbrooke’s Cognitive Examination-Revised (ACE-R)) was included in the study. The participant's cognitive rehabilitation program consisted of a face-to-face training for twelve sessions and a daily homework-based training (35 days). The participant was tested at the baseline, at the end of the rehabilitation procedure, and 6- and 12-month follow-ups. To measure memory, attention, executive function, language, and reasoning abilities the following tests were administered: (a) Forward and Backward Digit Span Test; (b) Rey Complex Figure Test; (c) Continuous Performance Test; (d) Color-Word Interference; (e) Verbal Fluency; (f) Trial Making Test; (g) Symbol Digit Modality Test; and (h) The Twenty Questions Test and the Word Context Test. The Mean Percentage Improvement (MPI) index suggested improvements in the participant's scores for ACE-R subscales, memory, attention, language, executive functions, and reasoning and persistent improvements in the Word Context Test (55% at post-test and 63% at follow-up) and Rey Complex Figure Test (33% at the post-test and 47% at the follow-up). The results suggest that the CMP could improve the overall cognitive functioning of the participant.
Keywords: Mild Neurocognitive Disorder; Dementia; Alzheimer; Cognitive Rehabilitation reduction
Abbreviations: NCD: Neurocognitive Disorder, CMP: Cognitive Management Program, ACE-R: Addenbrooke’s s Cognitive Examination-Revised, MPI: Mean Percentage Improvement, MCI: Mild Cognitive Impairment, MMSE: Mini-Mental State Examination, GDS: Geriatric Depression Scale, D-KEFS: Delis-Kaplan Executive Function System, TMT: Trial Making Test, MPI: Mean Percentage Improvement
Introduction
Mild cognitive impairment (MCI) is a common diagnosis among older adults [1,2]. Due to the importance of MCI, DSM-5 introduced a new diagnostic framework for screening mild neurocognitive disorders (mild-NCD) [3]. Mild-NCD is defined as a deficit in at least one cognitive function that has not been evident in the person's performance in the past. In addition, the deficit should not be a result of other forms of delirium and cannot be explained by other mental disorders [4,5]. The first subtype of mild-NCD is mild neurocognitive disorder caused by Alzheimer (mild-NCD due to Alzheimer), which is characterized by a progressive memory loss and learning inability. However, there are other subtypes that can describe cognitive dysfunction including mild vascular neurocognitive disorder or mild neurocognitive disorder due to HIV infection [4].
Precise diagnosis is the first and the most important phase in the management of cognitive disorders in individuals with cognitive disorders like mild-NCD. To understand the nature of the disorder, it is necessary to manage its symptoms, alleviate its consequences, and prepare a multi-dimensional cognitive profile [6]. Moreover, a detailed cognitive neuropsychological assessment with a compressive emphasis on specific cognitive domains (i.e., memory, attention, language, executive functions, and reasoning) can provide a more inclusive evaluation prior to the rehabilitative intervention [7]. For instance, the diagnosis of attention deficit, which adversely affects memory [8], is possible only through a multi-dimensional cognitive assessment.
Although multi-dimensional assessment and cognitive rehabilitation with a specific emphasis on real life performance can provide promising results [9,10], there is not a general consensus over procedures through which cognitive rehabilitation can assist individuals with mild-NCD [10]. To develop a comprehensive model of cognitive rehabilitation for individuals with cognitive disorders, more evidence-based research is essential [11]. Thus, this study focuses on an evidence-based CMP that comprises three components: (a) diagnosis, (b) assessment, and (c) cognitive rehabilitation.
Case Illustration
The present study addresses issues regarding diagnosis, assessment, and cognitive rehabilitation procedures in a right-handed [12], literate, 59-year-old woman who lived with her husband and daughter. Her medical records suggested that she had mild neurocognitive disorder (mild-NCD) caused by Alzheimer and was recruited from a daily Nursing Home in Mashhad-Iran. Her chief complains were related to memory problems, e.g., forgetting daily routines like taking medications. She had trouble remembering the name of people she had just met. She complained that she could not learn new things and had therefore withdrawn from her English language classes (a curriculum held by the Nursing Home). Likewise, she had trouble arranging her daily activities, and could not plan for participating in regular schedules organized by the Nursing Home. She reported that she does not care much about her diet or sleeping schedule. She suffered from mild knee arthritis and took medication to control urinary incontinence, which was interrupting her sleep. In addition, she took low-dose fluoxetine for years to manage her mood. However, she did not meet the criteria for depression disorder.
Methods
Diagnosis
The participant met [at the baseline] the DSM-5 criteria for mild neurocognitive disorder caused by Alzheimer. On Addenbrooke’s Cognitive Examination-Revised (ACE-R) test, she gained a score of 78 that was suggestive of a cognitive state between MCI and Alzheimer. In keeping with her complains and compared with her scores on attention and language tasks, she gained the lowest score on memory performance task (score = 14). (Table 1).
To arrive at a valid diagnosis, other subtypes of mild-NCD were also evaluated based on DSM-5 criteria and the participant’s medical records and laboratory findings. In addition, the participant's scores on the geriatric depression scale suggested that her scores on the cognitive tasks were not an artifact of depression at all assessment points.
To develop a satisfactory cognitive performance profile, a multi-dimensional battery of cognitive functioning (i.e., memory, attention, verbal fluency, executive functions, and reasoning) was used in all assessment points (Table 2).
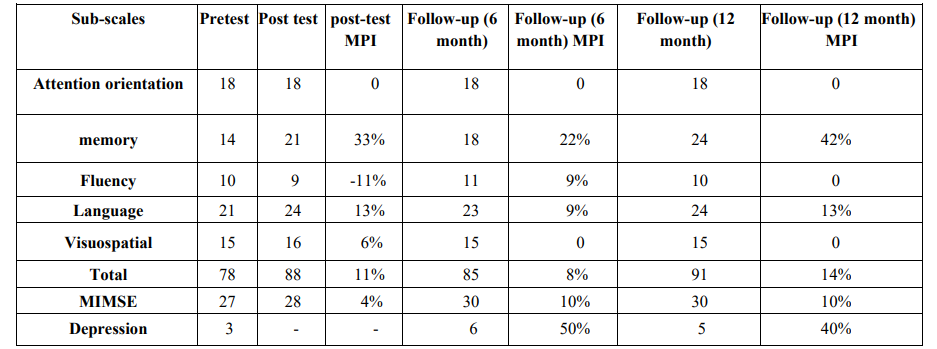
Table 1: Shows attention-orientation, memory, fluency, language, visuospatial, total score, MIMSE, depression in each stage at pre-test, post-test, and at 6 and 12-month follow-ups.
Note. Mean percentage improvement (MPI) was used to show the magnitude of the effect.
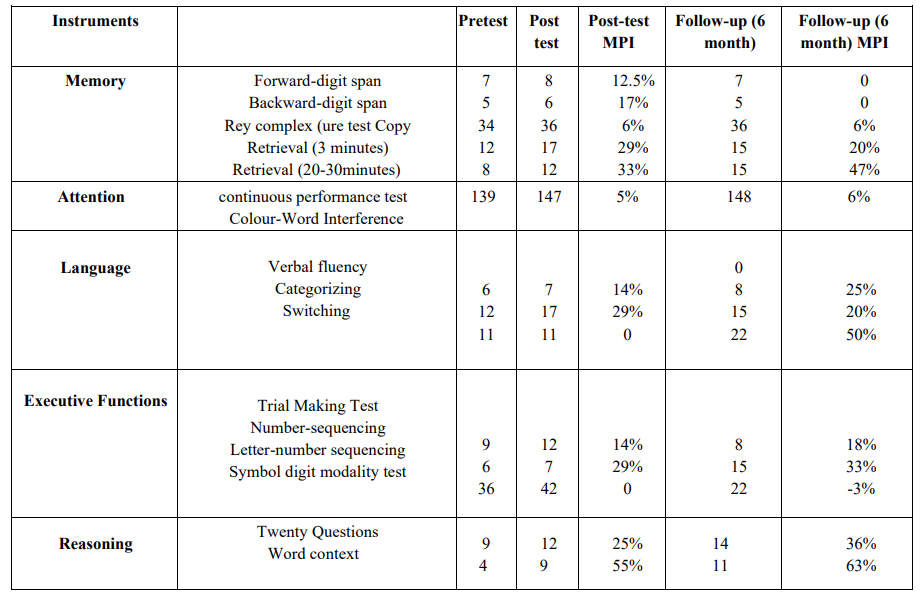
Table 2: Memory, Attention, executive function, language and reasoning subject's scores in the pre-test, post-test, and follow-up.
Note. mean percentage improvement (MPI) have been used to show magnitude of effect.
Intervention
A cognitive rehabilitation program was designed based on the diagnostic criteria and the outcomes of the multi-dimensional cognitive assessment with an emphasis on memory and tasks of executive functions (Table 3). With some modifications, the rehabilitation program consisted of several practices adapted from previous studies [13,14]. Cognitive rehabilitation was divided into two types of training: (a) an in-person training (through face-to-face sessions); and (b) a home-based training (see Table 3). The former consisted of two to three sessions (90-min) per week (a total of twelve sessions), and the latter was performed every day by the participant; each session was monitored by the experimenter on the same day. The cognitive intervention lasted for 35 days. The rationale behind home-based training was that some tasks like diary training and executive function training strategies (e.g., planning skills) can best be promoted through daily practices by an individual at home [15].
The participant was provided with sufficient, clear instructions on how to complete home-based training to improve her memory and manage her daily routines. The instructions covered four areas: (a) direction or orientation [13], (b) a daily timetable, (c) diary writing, (d) writing of new items or a specific piece of information that she was expected to learn and remember in the next session [14, 16-18].
Daily face-to-face sessions were arranged with the participant to overview home-based training and to evaluate her progress with the previous session exercises. Different types of reasoning-based practices (categorization, summarization, and comprehension strategies) (Table 3) were scheduled for the initial phase of the intervention to enhance learning processes trailed by memory training [19;21]. There is evidence that improvements in memory can, in turn, enhance reasoning skills [22-24]. Additional trainings were included in the program to improve the participant's attention, executive functions, and language capabilities (i.e., fluency, switching, and categorization).
Procedure
A written informed consent was obtained from the participant prior to the study. The present study lasted from January 2019 until March 2020 (follow-up). One of the present coauthors was responsible for the administration of the study. She was granted access to all medical records of the participant in the Nursing Home. The diagnostic procedure was carried out by the experimenter who holds a degree in psychology and a gerontologist at the Nursing Home. All testing and intervention sessions were conducted in a silent room at the Nursing Home. The room was furnished with comfortable seats and an office desk. The second 12-month follow-up partly coincided with the outbreak of COVID-19 pandemic and we were not able to administer all post-test measures due to the pandemic. Therefore, only tests of diagnostic criteria (i.e., ACE-R and GDS) for the participant's cognitive abilities are reported at the 12-month follow-up.
Instruments
Diagnostic tests
Addenbrooke’s Cognitive Examination Revised (ACE-R)
First, the Persian version of Addenbrooke’s Cognitive Examination-Revised (ACE-R) was administered with the participant. The ACE-R is an extension of the Mini-Mental State Examination (MMSE) [25] and has good reliability and validity [26,27]. The participant scored 78 on the ACE-R. A score of 78 and lower is suggestive of Alzheimer's disease whereas scores between 78-85 are suggestive of MCI.
Geriatric Depression Scale (GDS)
The participants' depression symptoms were assessed using the validated and reliable Persian version of GDS [28-30]. A score of ≥ 8 indicates clinically relevant depression, which was not met by the present participant.
Cognitive Battery Tests

Figure 3: Personal (face to face) and home-based training.
Note. * shows the types of cognitive rehabilitation
Memory
Tests of short-term, long-term, and working memory, forward and backward digit span (adapted from the Wechsler Memory Scale) [31] and Rey-Osterrieth Complex Figure Test (i.e., copy, immediate recall, and delayed recall) [32] were administered with the participant, respectively.
Attention
Computer-based Continuous Performance Test [33] was used to measure sustained attention. Color-Word Interference Test was administered to measure inhibition and switching [34]. This test was extracted from the Persian version of the Delis-Kaplan Executive Function System (D-KEFS) [35].
Executive function
The executive functions were measured using Symbol-Digit Modality Test (S-DMT) (36) and Trial Making Test (TMT) [35,36].
Language
D-KEFS subtests of verbal fluency, categorization, and switching standardized for Persian language were administered with the participant to measure her language abilities [35,36].
Reasoning
D-KEFS subtest of Twenty-Question Tests and Word Context were used to measure the participant's reasoning skills [35,36].
Data analysis
To analyze data on diagnosis and assessments, we used Trend Change Chart and Mean Percentage Improvement (MPI) index. The MPI index is a measure of clinical significance that is used to show the mean improvement percent, which is calculated from the following equation:
MPI = [(baseline mean – treatment phase mean) / treatment phase mean] × 100
Results
The following presents the study results for diagnostic criteria and cognitive performance. In terms of diagnostic criteria, the results for the ACE-R sub-scales indicated improvements in scores for language, memory, and visuospatial skills from pre-test to post-test and to the 6- and 12-month follow-ups (Table 1). The total ACE-R score increased to the normal range (88-100) at the post-test, with a minor decrease at the 6-month follow-up (85), and again a major spike at 12-month follow-up (91) (Table 1).
Moreover, the highest improvement was observed in the memory subscale at the post-test (33%), at the 6-month follow-up (22%), and at the 12-month follow-up (42%). It was followed by improvements in the language subscale at the post-test (13%), at the 6-month follow-up (9%), and at the 12-month follow-up (13%) (Table 1).
In terms of multi-dimensional cognitive functioning (Table 2), there was a 55% increase at the post-test and a 63% increase at the 6-month follow-up scores for Word Context Test. Memory in the Rey-Osterrieth Complex Figure Test's delayed recall subscale was the next variable with 33% and 47% improvements at the post-test and the 6-month follow-up, respectively. Further increases were observed at the post-test for the 3-min immediate recall (29%) and for the categorization of language (29%); the increase in both indices was smaller at the follow-up assessment (i.e., 20% of increase compared with the pre-test assessment). All language subscales except improved substantially at the post-test and follow-ups. Among attention subscales, only switching and Continuous Performance Test showed an improvement. All subscales of executive functions improved at the post-test (Table 2). No complications or undesired side effects were reported by the participant, the experimenters or the nursing home team members.
Discussion
The present study aimed to diagnose, assess, and intervene in the process of mild neurocognitive disorder (mild-NCD) caused by Alzheimer. Our findings suggest substantial improvements in the cognitive performance of an individual with mild-NCD, as evident from increases in her scores from pre-test to post-test and the follow-up assessments for the following indices: short-term and working memory, visual memory, sustained attention, switching, verbal fluency, categorization, number-sequencing, letter-number sequencing, symbol digit modality, twenty questions, and word context.
Considering the need for categorization, comprehension, summarization, and structuring information, reasoning improved smoothly during the training and reached a high level for both auditory and visual tasks. In reciprocity, memory training would help improve reasoning and language abilities. Accordingly, there is evidence supporting the strong relationship among language, memory, and reasoning [22-24, 37-40]. Each of the above can have a positive effect on one another, which should be considered in a rehabilitation model. In addition, the participant reported that when she intended to participate in the Nursing Home’s book club, she tried to categorize information by taking notes and remembering them later. The participant reported a similar improvement in the same way (i.e., categorization, preparation, and finalization) by drawing a picture in the Rey test.
Likewise, the participant’s ability to plan her daily activities improved, and she was able to manage training, which called for a satisfactory level of planning (e.g. planning a travel for tour members by considering limitations such as the timetable of activities, traveler’s preferences, and several such factors). Consistent with her progress in planning, her scores improved in the subscales of executive functions (see Table 2).
Our findings provide support for the view that cognitive-based therapies may improve cognitive functions [41]. However, the extent of improvement may vary in various cognitive domains. In the present study, the highest improvements were observed in the participants' language capabilities (except for verbal fluency), working memory, and visuospatial skills which further support findings reported by Spector et al [42] and Hall et al. [42].
The main assumption underlying cognitive-based interventions is that cognitive rehabilitation can reverse cognitive decline in individuals at risk of Alzheimer [43]. the rehabilitation can exerts its impact through neurocognitive mechanisms, including (a) adaptation; (b) cognitive control; and (c) categorization through organization [44]. Another explanation is that adhering to a home-based training (i.e., directions questions, diary writing, and checking a timetable on a daily basis) reduces stress, and improves learning and memory [45-47].
Likewise, reasoning training (e.g., categorization) and language practices (speaking for a limited time) can expedite the cognitive rehabilitation process [48]. Most of the changes (i.e., restorations and improvements) appear to be the outcomes of effective and organized behavioral adaptive coding in the prefrontal cortex and reciprocal, network-related cortico-cortical and cortico-subcortical connectivity and activities [44,49].
Although the present study focused on a real-life training (Table 3), there are some limitations associated with ecological rehabilitation and the generalization of the skills to other real-world situations [50]. The limitation seems to be due to the complex relationship between everyday activities and cognitive domains that they engage [16]. Therefore, future studies can explore these relationships to develop more comprehensive real-life training programs, which could involve using computerized technologies.
To conclude, memory, language, and reasoning appear to play a substantial role in the success of cognitive management program in individuals at risk of developing dementia caused by Alzheimer's disease. Careful diagnosis of mild-NCD, followed by a multi-dimensional assessment of the clients' cognitive functioning, could promote evidence-based findings that are necessary to develop effective cognitive-based intervention [51].
Acknowledgement
We wish to express our sincere gratitude to the editor and anonymous reviewers of this manuscript who assisted us with their invaluable comments. We also offer special thanks to our participant who willingly took part in the present study. We have no conflict of interest to declare.
Fundings
The present study was partially supported by a fund to the first and second authors (FUM-50736).
Ethical Committee Registration Number:
IR.UM.REC.1399.031
The Ethics Committee of Mashhad University of Mashhad approved the study. participant gave informed consent before participating.
References
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, et al. (1997) Aging, memory, and mild cognitive impairment. Int Psychogeriatr. 9(Suppl 1): 65-69. [PubMed.]
- Blazer D. (2013) Neurocognitive disorders in DSM-5. American Journal of Psychiatry. 170(6): 585-587. [PubMed.]
- Ganguli M. (2013) Can the DSM-5 framework enhance the diagnosis of MCI? Neurology. 81(23): 2045-2050. [Ref.]
- Association AP. (2013) Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub). [Ref.]
- Sachs-Ericsson N, Blazer DG. (2015) The new DSM-5 diagnosis of mild neurocognitive disorder and its relation to research in mild cognitive impairment. Aging & mental health. 19(1): 2-12. [PubMed.]
- Bayley MT, Tate R, Douglas JM, Turkstra LS, Ponsford J, et al. (2014) INCOG guidelines for cognitive rehabilitation following traumatic brain injury: methods and overview. The Journal of head trauma rehabilitation. 29(4): 290-306. [PubMed.]
- Woods B, Aguirre E, Spector AE, Orrell M. (2012) Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database of Systematic Reviews. (2). [PubMed.]
- Luo X, Guo J, Liu L, Zhao X, Li D, et al. (2019) The neural correlations of spatial attention and working memory deficits in adults with ADHD. NeuroImage Clinical. 22: 101728. [PubMed.]
- Wilson BA. (2002) Towards a comprehensive model of cognitive rehabilitation. Neuropsychological rehabilitation. 12(2): 97-110. [Ref.]
- Kelly ME, O'Sullivan M. (2015) Strategies and techniques for cognitive rehabilitation: manual for healthcare professionals working with individuals with cognitive impairment. [Ref.]
- Huckans M, Hutson L, Twamley E, Jak A, Kaye J, et al. (2013) Efficacy of cognitive rehabilitation therapies for mild cognitive impairment (MCI) in older adults: working toward a theoretical model and evidence-based interventions. Neuropsychology review. 23(1): 63-80. [PubMed.]
- Oldfield RC. (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 9(1): 97-113. [PubMed.]
- Brum PS, Forlenza OV, Yassuda MS. (2009) Cognitive training in older adults with mild cognitive impairment: Impact on cognitive and functional performance. Dementia & Neuropsychologia. 3(2): 124-131. [Ref.]
- Wilson BA. (2009) Memory rehabilitation: Integrating theory and practice. Guilford Press. [Ref.]
- Stamenova V, Levine B. (2018) Effectiveness of goal management training® in improving executive functions: A meta-analysis. Neuropsychological rehabilitation. 29(3): 1-31. [Ref.]
- Levaux M-N, Fonteneau B, Larøi F, Offerlin-Meyer I, Danion J-M, et al. (2012) An individualized and everyday life approach to cognitive rehabilitation in schizophrenia: a case illustration. Rehabilitation research and practice 2012. 928294. [Ref.]
- Wilson BA, Winegardner J, van Heugten CM, Ownsworth T. (2017) Neuropsychological rehabilitation: The international handbook: Psychology Press. [Ref.]
- Yang C, Potts R, Shanks DR. (2018) Enhancing learning and retrieval of new information: a review of the forward testing effect. NPJ science of learning. 3(1): 1-9. [Ref.]
- Constantinidou F, Thomas RD, Robinson L. (2008) Benefits of categorization training in patients with traumatic brain injury during post–acute rehabilitation: additional evidence from a randomized controlled trial. The Journal of head trauma rehabilitation. 23(5): 312-328. [PubMed.]
- Spirgel AS, Delaney PF. (2016) Does writing summaries improve memory for text? Educational Psychology Review. 28(1): 171-196. [Ref.]
- Thorndyke PW. (1977) Cognitive structures in comprehension and memory of narrative discourse. Cognitive psychology. 9(1): 77-110. [Ref.]
- Beatty EL, Vartanian O. (2015) The prospects of working memory training for improving deductive reasoning. Frontiers in human neuroscience. 9: 56. [Ref.]
- Ariës RJ, Groot W, van den Brink HM. (2015) Improving reasoning skills in secondary history education by working memory training. British Educational Research Journal. 41(2): 210-228. [Ref.]
- Carretti B, Caldarola N, Tencati C, Cornoldi C. (2014) Improving reading comprehension in reading and listening settings: The effect of two training programmes focusing on metacognition and working memory. British Journal of Educational Psychology. 84(2): 194-210. [Ref.]
- Hodges JR, Larner AJ. (2017) Addenbrooke’s cognitive examinations: Ace, ace-r, ace-iii, aceapp, and mace. Cognitive screening instruments: Springer). 109-137. [Ref.]
- Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. (2006) The Addenbrooke's Cognitive Examination Revised (ACE‐R): a brief cognitive test battery for dementia screening. International Journal of Geriatric Psychiatry: A journal of the psychiatry of late life and allied sciences. 21(11): 1078-1085. [PubMed.]
- Pouretemad HR, Khatibi A, Ganjavi A, Shams J, Zarei M. (2009) Validation of Addenbrooke’s cognitive examination (ACE) in a Persian-speaking population. Dementia and geriatric cognitive disorders. 28(4): 343-347. [PubMed.]
- McGivney SA, Mulvihill M, Taylor B. (1994) Validating the GDS depression screen in the nursing home. Journal of the American Geriatrics Society. 42(5): 490-492. [PubMed.]
- Lesher E. (1986) Validation of the Geriatric Depression Scale among nursing home residents. Clinical Gerontologist. 4(4): 21-28. [Ref.]
- Malakouti K, Fathollahi P, Mirabzadeh A, Salavati M, Kahani S. (2006) Validation of geriatric depression scale (GDS-15) in Iran. Research in Medicine. 30(4): 361-369. [Ref.]
- Wechsler D, Scale WAI. (1981) Revised. Psychological Corp, San Antonio, TX. [Ref.]
- Shin M-S, Park S-Y, Park S-R, Seol S-H, Kwon JS. (2006) Clinical and empirical applications of the Rey- Osterrieth complex figure test. Nature protocols. 1(2): 892-899. [PubMed.]
- Rosvold HE, Mirsky AF, Sarason I, Bransome Jr ED, Beck LH. (1956) A continuous performance test of brain damage. Journal of consulting psychology. 20(5): 343-350. [PubMed.]
- Ghawami H, Raghibi M, Tamini BK, Dolatshahi B, Rahimi-Movaghar V. (2016) Cross-cultural adaptation of executive function tests for assessments of traumatic brain injury patients in southeast Iran1. Psicología Conductual. 24(3): 513-554. [Ref.]
- Delis DC, Kramer JH, Kaplan E, Holdnack J. (2004) Reliability and validity of the Delis-Kaplan Executive Function System: an update. Journal of the International Neuropsychological Society: JINS. 10(2): 301-103. [PubMed.]
- Smith A. (1973) Symbol digit modalities test: Western Psychological Services Los Angeles. [Ref.]
- Heit E, Rotello CM, Hayes BK. (2012) Relations between memory and reasoning. Psychology of learning and motivation. Elsevier. 57: 57-101. [Ref.]
- Brainerd CJ, Reyna VF. (2001) Fuzzy-trace theory: Dual processes in memory, reasoning, and cognitive neuroscience. [PubMed.]
- Burke DM, MacKay DG. (1997) Memory, language, and ageing. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 352(1363): 1845-1856. [Ref.]
- Carretti B, Borella E, Zavagnin M, de Beni R. (2013) Gains in language comprehension relating to working memory training in healthy older adults. International journal of geriatric psychiatry. 28(5): 539-546. [PubMed.]
- Jeong JH, Na HR, Choi SH, Kim J, Na DL, et al. (2016) Group-and home-based cognitive intervention for patients with mild cognitive impairment: a randomized controlled trial. Psychotherapy and Psychosomatics.85(4): 198-207. [PubMed.]
- Hall L, Orrell M, Stott J, Spector A. (2013) Cognitive stimulation therapy (CST): neuropsychological mechanisms of change. International Psychogeriatrics. 25(3): 479-489. [PubMed.]
- Swaab D, Dubelaar E, Hofman M, Scherder E, Van Someren E, et al. (2002) Brain aging and Alzheimer's disease) use it or lose it. Progress in brain research. Elsevier. 138: 343-373. [PubMed.]
- Duncan J. (2001) An adaptive coding model of neural function in prefrontal cortex. Nature reviews neuroscience. 2(11): 820-829. [PubMed.]
- Sandi C. (2007) Chapter 12 Memory Impairments Associated with Stress and Aging. Neural plasticity and memory: From genes to brain imaging. 225. [Ref.]
- Murnahan B. (2010) Stress and Anxiety Reduction Due to Writing Diaries, Journals, E-mail, and Weblogs. [Ref.]
- Clare L, Woods B. (2003) Cognitive rehabilitation and cognitive training for early‐stage Alzheimer's disease and vascular dementia. Cochrane database of systematic reviews. 4. [PubMed.]
- Wilson BA. (2003) Memory rehabilitation. Neuropsychology of Memory. 263. [Ref.]
- Mateer CA. (2005) Fundamentals of cognitive rehabilitation. Effectiveness of rehabilitation for cognitive deficits. 21: 29. [Ref.]
- Hampstead BM, Gillis MM, Stringer AY. (2014) Cognitive rehabilitation of memory for mild cognitive impairment: a methodological review and model for future research. Journal of the International Neuropsychological Society. 20(2): 135-151. [PubMed.]
- Bahar-Fuchs A, Clare L, Woods B. (2013) Cognitive training and cognitive rehabilitation for persons with mild to moderate dementia of the Alzheimer's or vascular type: a review. Alzheimer's research & therapy. 5(4): 35. [PubMed.]