>Corresponding Author : Tabat Meryem
>Article Type : Case Report
>Volume : 3 | Issue : 6
>Received Date : 02 Sep, 2023
>Accepted Date : 11 Sep, 2023
>Published Date : 20 Sep, 2023
>DOI : https://doi.org/10.54289/JCRMH2300127
>Citation : Karim M, Meryem T, Marouane S, Zineb EJ, Haboub M, et al. (2023) A Preoperative Evaluation Revealing a Rare Coagulation Factor Deficiency. J Case Rep Med Hist 3(6): doi https://doi.org/10.54289/JCRMH2300127
>Copyright : © 2023 Karim M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access
Department Of Cardiology. Ibn Rochd University Hospital of Casablanca, Morocco
*Corresponding author: Tabat Meryem, Department Of Cardiology. Ibn Rochd University Hospital of Casablanca, Morocco
Abstract
Constitutional factor II deficiency is a rare coagulation disorder. The autosomal recessive form has an estimated prevalence of 1-2 million. Clinical symptoms depend on the rate of factor II deficiency.
We report a fortuitous discovery of an isolated factor II deficiency in a 9-year-old girl of 1st degree consanguineous parents, following a preoperative assessment of drainage of her abundant pericardial effusion, the biological assessment showed a factor II rate < 1%, a prothrombin rate at 3% and a TCA > 120 sec, the dosage of the other coagulation factors are without anomalies.
Through this observation we recall the rarity of this deficit and thought about it in the face of unusual bleeding in newborns of consanguineous parents, as well as the necessity of collaboration with international registries to establish codified diagnostic and therapeutic guidelines.
Keywords: Factor II; Congenital Hemorrhagic Diseases; Rare Coagulation Factor Deficiency
Abbreviations: FFP: Fresh Frozen Plasma
Introduction
Coagulation factor II or prothrombin, is vitamin k dependent. Acquired prothrombin deficiency occurs in patients with vitamin K deficiency, liver disease, patients on vitamin K antagonists, or the presence of lupus-anticoagulant hypothrombin syndrome [1]. Hereditary prothrombin deficiency is one of the rarest hemostasis disorders, it has an autosomal recessive transmission with an occurrence of 1 in 1 to 2 million people [2]. We report in our observation the case of a fortuitous discovery of a constitutional factor II deficiency in a 6-year-old girl during a preoperative assessment for pericardial drainage, and we discuss the clinico-biological particularities of this rare deficiency., the different therapeutic modalities as well as the need for collaboration of the different international registries to establish well-codified replacement therapy.
Patient and observation
Patient information: A 08-year-old girl, the youngest of 3 siblings, from a 1st degree consanguineous marriage. The pregnancy was well monitred, the delivery was vaginally with no sign of natal suffering, the patient was breastfed, she was well vaccinated according to the national immunization program, with no other surgical, medicam or allergic history. The patient presented with progressive dyspnea and her parents presented to the emergency departement.
Clinical findings: The clinical examination finds a conscious patient, normocoloured, with a good height and weight development, polypneiques at 34 cycles/min, without signes of respiratory struggles, the oxygen saturation in the oprn air air was at 95%, the heart rate was at 95%, the heart rate was at 116 bats per min without angina or palpitations cardiac auscultation finds a muffled heart sound, without externalized bleeding.
Diagnostic assessment: A chest X ray was dose, objectifying cardiomegaly with a cardiothoracic index of 0, 8 (figure 1). An ECG was done showing microvoltage at the peripheral leads (figure2). A thransthoracic echocardiography also done showing a large pericardial effusion with the following measurements: Right ventricle: 33mm ;Right atrium: 34mm; left ventricle:41mm ;left atrium:39mm (figure 3). Inferior vena cava at 19 mm compliant, without signs of tamponade. The indication for surgical drainage with pericardial biopsy was posed . the preoperative assessement showed a distrubed haemostasis assessment with a PT=3%, TCK=120sec, Factor V=70%, Factor X=129%, fibrinogene at 3, 44. A sd assessement was resquested objectifying an isolated deficiency in factor II. Hepatic and renal assessement was correct, as well as the rest of the etiological assessement of the effusion pericardium was unremarkables.
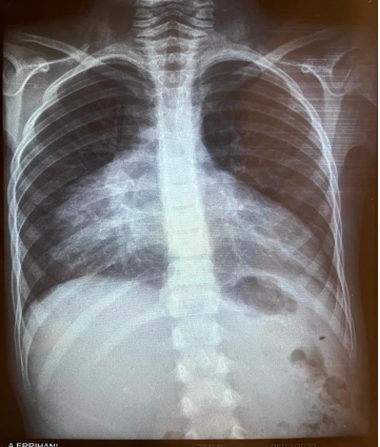
Figure 1: A chest X ray was dose, objectifying cardiomegaly with a cardiothoracic index of 0, 8.
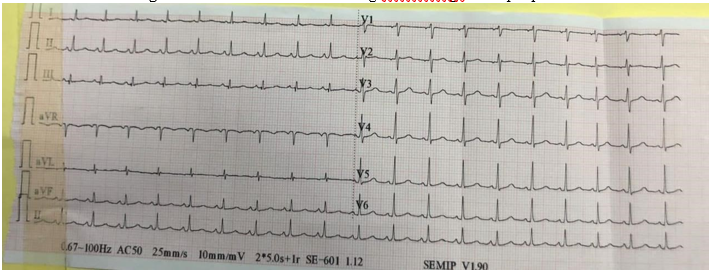
Figure 2: An ECG was done showing microvoltage at the peripheral leads.
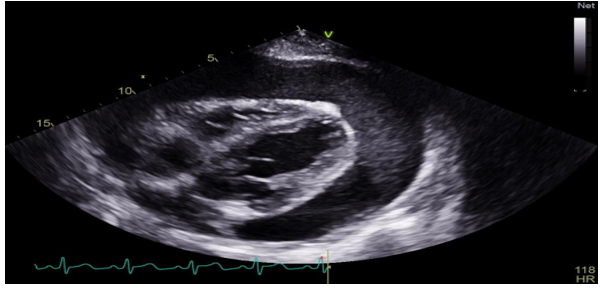
Figure 3: A thransthoracic echocardiography also done showing a large pericardial effusion.
Therapeutic interventions, Follow-up and outcome of interventions:
The patient benefited from a daily transfusion of fresh frozen plasma (FFP) at a rate of 20ml/kg /D ;after one week of transfusion assessment showing a PT=50% and TCK=35, 7 sec, pericardial drainage was performed bringing back a transudative fluid with culture on standard medium and Sabouraud were negative and on histological examination a fibrous reorganization and the Congo red coloration showing no deposit of amyloids, the postoperative follow-up was unremarkable, and the drain was ablated after 48 hours, and the ETT control showed a posterior effusion blade, a PFC transfusion was continued for two days; the patient was connected to a hematological consultation for follow-up.
Discussion
Prothrombin (factor II) deficiency is a rare coagulation disorder first reported by Quick in 1947 and further described in 1955 and 1962 [1]. Prothrombin deficiency can be acquired or inherited.
The hereditary form is extremely rare with a prevalence of approximately 1 in 2 million in the general population, only about thirty cases of congenital factor II deficiency have been documented worldwide [3]. Its prevalence is higher in the population resulting from a consanguineous marriage which is the case of our patient resulting from a consanguineous marriage in the 1st degree [4,5]. Measuring plasma levels of prothrombin as the functional activity or immunoreactive protein, two main phenotypes can be distinguished: (1) hypoprothrombinaemia (type I deficiency), characterized by concomitantly low levels of activity and antigen; and (2) dysprothrombinaemia (type II deficiency), characterized by the normal synthesis of a dysfunctional protein (low coagulant activity but normal or borderline antigen levels [6]. The severity of deficiency was classified according to factor II level:• Severe deficiency: factor II 1%. Moderate deficiency: factor II 1% < level < 5%. Minor deficiency: factor II > 5% [3]. Hypoprothrombinemic patients may present with prolonged post-injury bleeding, mucosal bleeding, hematomas and hemarthroses; whereas patients with measurable levels of prothrombin > 30/40% or dys-prothrombinemia may remain asymptomatic [7]. Since 1947, no progression has occurred in the development of factor II concentrates as there are few cases reported till now. The development in this way is very slow probably because of the cost-benefit reasons especially with current financial limitations as well as the rarity of cases worldwide. There are neither clear guidelines on prophylaxis nor on treatment [8]. Acute bleeding episodes can be treated with fresh frozen plasma (FFP) at 15-20 mL kg)1, which will usually raise the factor level by 25%.for surgical procedures or more severe bleeding episodes, a loading dose of 15-20 mL kg)1 of fresh frozen plasma (FFP) followed by 3 mL kg every 12-24 h is usually adequate for haemostasis [1] our patient received a loading dose of 15ml / kg of PFC with a maintenance dose of 10 ml / kg, the postoperative follow-up was without anomalies, the drain brought back serohematic fluid, and was ablated after 24 hours of the procedure. The exact level of prothrombin required for hemostasis is not known, but 10-15% may be sufficient to prevent minor bleeding and 20-40% for major trauma or surgery [2]. The prothrombin complex at a dose of 20-30ui/kg based on the units of factor IX in the product used [8]. Anti-fibrinolytic therapies such as trexanic acid can be administered intravenously or orally, in patients with mild-to-moderate deficiency, to control epistaxis, menorrhagia, or other non-lifethreatening mucosal bleeding [9] hormonal therapy with estrogens and/or progesterone may help to reduce menstrual blood loss in patients with menorrhagia [8].
Conclusion
Prothrombin deficiency is the rarest of hemostasis disorders, clinical manifestations can be minimal or very important with life-threatening bleeding. Current treatment guidelines are based on personal experience or expert recommendations, given the small number of patients with this deficit, it is essential to collaborate with international registries for better diagnostic and therapeutic management.
Informed consent and patient consent: written consent was obtained from the patient to publish images and clinical information relating to the case. The patient consented to his clinical information except name to be published to contribute to science and global health.
Authors' contributions:
Karim Mounaouir: patient management, data collection and analysis.
Tabat Meryem: paper preparation.
Salmaoui Marouane and El Jaouhari Zineb: manuscript writing and revision.
All the authors have read and agreed to the final manuscript.
Competing interests:
The authors declare no competing interests
References
- Meeks SL, Abshire TC. (2008) Abnormalities of prothrombin: a review of the pathophysiology, diagnosis, and treatment. Haemophilia. 14(6): 1159-1163. [PubMed.]
- Lancellotti S, De Cristofaro R. (2009) Congenital prothrombin deficiency. Semin Thromb Hemost. 35(4): 367-381. [PubMed.]
- Imane S, Laalej Z, Faez S, Oukkache B. (2013) Congenital factor II deficiency: moroccan cases. Int J Lab Hematol. 35(4): 416-420. [PubMed.]
- Jaouad IC, Elalaoui SC, Sbiti A, Elkerh F, Belmahi L, et al. (2009) Consanguineous marriages in Morocco and the consequence for the incidence of autosomal recessive disorders. J Biosoc Sci. 41(5): 575-581. [PubMed.]
- Mannucci PM, Duga S, Peyvandi F. (2004) Recessively inherited coagulation disorders. Blood. 104(5): 1243-1252. [PubMed.]
- Peyvandi F, Duga S, Akhavan S, Mannucci PM. (2002) Rare coagulation deficiencies. Haemophilia. 8(3): 308-321. [PubMed.]
- Menegatti M, Peyvandi F. (2019) Treatment of rare factor deficiencies other than hemophilia. Blood. 133(5): 415-424. [PubMed.]
- Albalawi MA. (2019) Prothrombin (Factor II) deficiency as a rare bleeding disorder. JAPER. 9(4). [Ref.]
- Palla R, Peyvandi F, Shapiro AD. (2015) Rare bleeding disorders: diagnosis and treatment. Blood. 125(13): 2052-2061. [PubMed.]