>Corresponding Author : Mohammed Assaad Alnafie
>Article Type : Case Report
>Volume : 3 | Issue : 8
>Received Date : 28 Nov, 2023
>Accepted Date : 11 Dec, 2023
>Published Date : 15 Dec, 2023
>DOI : https://doi.org/10.54289/JCRMH2300140
>Citation : Alnafie MA, Moualek S and Ghebriout B. (2023) Incidental Postmortem Diagnosis of Anterior Communicating Artery Aneurysm After Successful Treatment of Double Aneurysms of the Superior Cerebellar and Paraclinoid Internal Carotid Arteries. J Case Rep Med Hist 3(8): doi https://doi.org/10.54289/JCRMH2300140
>Copyright : © 2023 Alnafie MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access
1Faculty of Medicine of Oran, University of Oran 1-Ahmed Ben Bella, Algeria
2Department of Anatomy, University Hospital Center of Oran, Algeria
*Corresponding author: Mohammed Assaad Alnafie, Faculty of Medicine of Oran, University of Oran 1 Ahmed Ben Bella, Algeria
Abstract
Intracranial aneurysm diagnosis uses several tools, of which digital subtraction angiography is still the gold standard. Computed tomography angiography is widely used for its specificity and sensibility. The 3D slicer software is an interesting diagnosis tool that provides high-quality 3D modeling. It facilitates diagnosis and improves aneurysm assessment. However, it is still not approved for clinical tasks. We present a case of a woman treated for a ruptured paraclinoid and superior cerebellar artery aneurysms, and who succumbed to a post-procedural rebleeding. During a research routine, we realized a computed tomography angiography-based 3D slicer modeling that detected an incidental anterior communicating artery aneurysm. This case illustrates an unusual association of two rare intracranial aneurysm locations and the usefulness of the 3D slicer modeling tool in the diagnosis of intracranial aneurysms. Through this report, we recommend using this tool worldwide for clinical tasks.
Keywords: Subarachnoid hemorrhage; Aneurysm; Diagnosis; Computed tomography angiography; Digital subtraction angiography; 3D slicer
Abbreviations: SAH: Subarachnoid Hemorrhage, PCA: Posterior Cerebral Artery, SCA: Superior Cerebellar Artery, ACOA: Anterior Communicating Artery, CTA: Computed Tomography Angiography, DSA: Digital Subtraction Angiography, GCS: Glasgow Coma Scale, PCOA: Posterior Communicating Artery, ICA: Internal Carotid Artery
Introduction
Acute subarachnoid hemorrhage (SAH) is still associated with a high mortality rate, reaching 25%, and morbidity, with at least one-third of patients losing autonomy. Aneurysmal SAH is the most common non-traumatic form [1]. It represents a critical health problem since it results in 3-5% of strokes [2]. Anterior circulation aneurysms account for 80-90% of cases [1]. From them, paraclinoid aneurysms are potentially bleeding, have complex anatomical relationships, and are consequently difficult to manage [3]. Posterior circulation aneurysms, especially those of the posterior cerebral artery (PCA) and superior cerebellar artery (SCA), are uncommon and account for 10-20% of intracranial aneurysms [4,5,1]. They represent a surgical challenge with serious complications [4,5]. An unruptured aneurysm may still be asymptomatic, provoke compression symptoms, or lead to fatal and insidious SAH if ruptured. Anterior communicating artery (ACOA) aneurysms are the most common and often present complex morphology and blood flow conditions. Improving the management of aneurysmal SAH requires accurate diagnosis and perfect assessment of the aneurysm and its anatomical environment to enable adequate therapeutic planning. Brain aneurysm diagnosis is possible with conventional radiological exams such as computed tomography angiography (CTA), magnetic resonance angiography, and digital subtraction angiography (DSA) [3]. Even if DSA is the gold standard [1,3], CTA is widely used for aneurysm assessment thanks to its high sensitivity and specificity [2]. DSA is reserved for cases where the CTA does not give relevant information [6]. In addition, CTA is more adaptable to situations when surgery is urgently required, such as massive bleeding or cerebral herniation [1]. However, all these modalities provide 2D images. 3D models generated on 2D radiological images facilitate the diagnosis and improve radiological evaluation. It allows neurosurgeons to adapt their treatment strategy. The 3D slicer provides high-quality 3D modeling images. However, it is still used only for research and is not approved for clinical applications [3]. A 43-year-old woman underwent successful surgery for ruptured paraclinoid and superior cerebellar artery (SCA) aneurysms. However, she succumbed to an unexplained postoperative rebleeding. CTA-based 3D slicer modeling incidentally detected an ACOA aneurysm. We present this case for two purposes: reporting an exceptional association of a paraclinoid aneurysm and a PCA-SCA aneurysm, and highlighting the usefulness of 3D slicer modeling in the diagnosis and assessment of intracranial aneurysms.
Case report
A 43-year-old hypertensive woman visited the emergency department of her local community for a sudden onset of headaches. On the physical exam, she was hemodynamically stable with a blood pressure of 150/90 mm Hg. Her Glasgow Coma Scale (GCS) score was 15/15, and her pupils were isochoric and reactive to light and accommodation. No seizures, visual disturbance, sensorimotor deficit, or fever were noticed. A brain CT showed diffuse SAH with blood in basal cisterns, cerebellar tentorium, and temporal sulci associated with discreet brain edema (Figure 1). The SAH was graded as Hunt and Hess grade 1 and Fisher CT grade 3. Given brain CT results and hypertensive peak, aneurysmal SAH was suspected. Thus, the patient was referred to the neurosurgery department. On admission, her GCS score was 14/15, and she had neck stiffness, photophobia, and phonophobia. In addition, left-sided ptosis and mydriasis were elicited, suggesting left oculomotor nerve palsy. The DSA was unavailable at our institution (a public hospital), and the cost was prohibitive at private facilities. So, a brain CTA with 3D reconstruction was performed, which revealed a 10 mm left paraclinoid aneurysm and a 9 mm left PCA aneurysm (Figure 2, Figure 3, Figure 4). The initial therapeutic strategy was maintaining systolic pressure between 120 and 140 mm Hg using an antihypertensive drug and preventing vasospasm using nimodipine. Since endovascular therapy was unavailable, surgeons chose surgical clipping. Therefore, the patient was transferred to the operating room, placed in the dorsal decubitus position, and the head right turned 60 degrees. Then, a left fronto-temporo-pterional craniotomy was performed. The paraclinoid aneurysm was oriented behind and was wide-necked. Its sac was bilobed and close to the pituitary fossa, the posterior communicating artery (PCOA), the superior wall of the cavernous sinus, and the left clinoid processes. Thus, the operating field was too narrow, and clipping might injure surrounding structures. Therefore, surgeons preferred muscle wrapping instead of clipping. The posterior aneurysm had a wide neck involving the left SCA and PCA origins and was close to the P1 and P2 segments. However, there was a good safety margin to clip the neck without interrupting the blood flow in the SCA and the PCA, which has been done. Despite successful surgery, the patient remained unconscious. On the sixth postoperative day, a brain CT was performed. It revealed a ventricular hemorrhage with diffuse cerebral edema, graded as Hunt and Hess 5 and Fisher CT grade 4. Thus, an external ventricular drain was urgently placed. Unfortunately, the patient succumbed to this event.
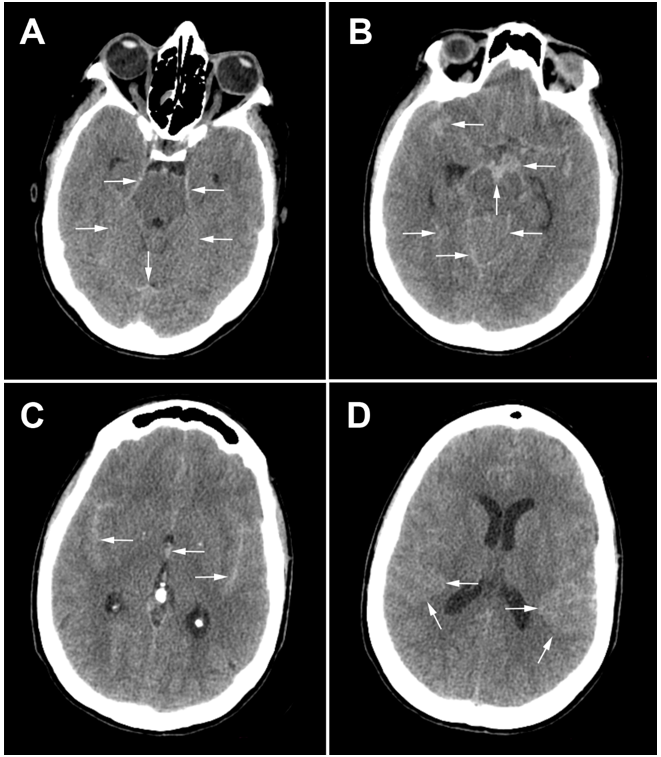
Figure 1.Brain CT scan slices: white arrows indicate the subarachnoid hemorrhage in basal cisterns, cerebellar tentorium, and temporal sulci.
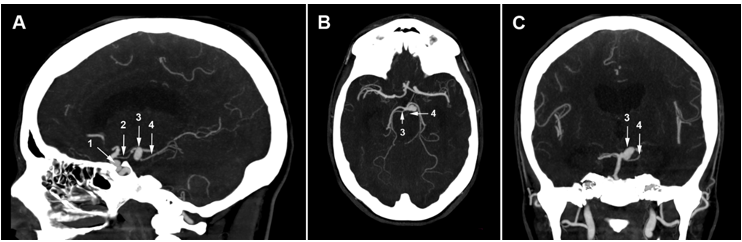
Figure 2.Sagittal, axial, and coronal slices of the brain CTA: the paraclinoid aneurysm (1) is close to the left clinoid processes and the left posterior communicating artery (2). The CTA shows a left posterior cerebral artery aneurysm (3). The origin of the left superior cerebellar artery (4) is not visible.
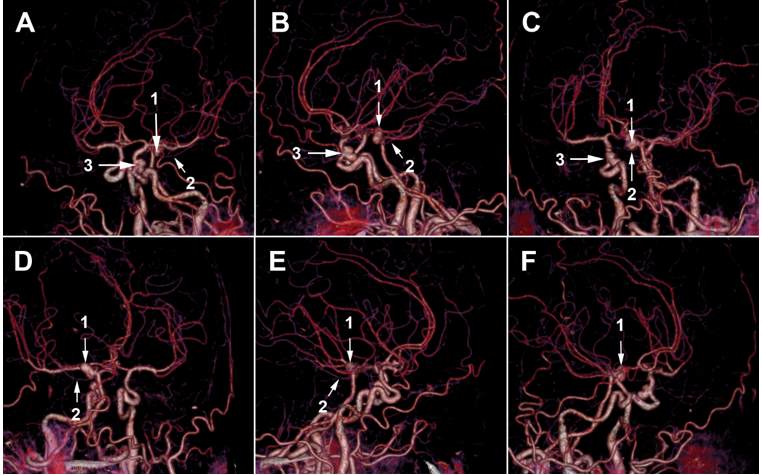
Figure 3.3D CTA images: we can see that the posterior aneurysm involves (1) the left posterior cerebral artery origin, but the origin of the left superior cerebellar artery (2) is still not clear. We can also see the paraclinoid aneurysm (3).
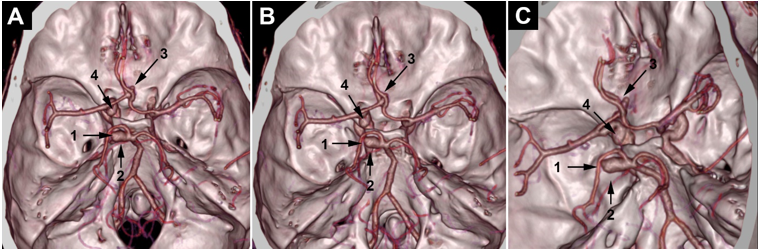
Figure 4.3D CTA volume rendering: the posterior aneurysm (1) involves the left posterior cerebral artery origin. The origin of the superior cerebellar artery (2) is still not clear. A little suspicious outpouching from the anterior communicating artery complex (3) is observed. The paraclinoid aneurysm is indicated in (4).
As a part of our research routine, we realized a retrospective CTA-based 3D modeling using the « 3D slicer » software. The 3D model’s results met the surgical findings concerning the paraclinoid and posterior aneurysm (Figure 5). In addition, it incidentally revealed a 5.2mm ACOA aneurysm (Figure 6). After discussion with neurosurgeons, the rebleeding was attributed to the rupture of the ACOA aneurysm.
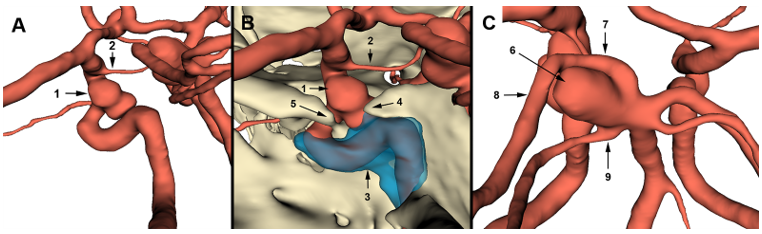
Figure 5.3D slicer modeling images: (A) the arterial model shows that the paraclinoid aneurysm (1) is of the inferior type according to Krisht and Hsu classification. The aneurysm is close to the posterior communicating artery (2). (B) Complete 3D representation of the paraclinoid region shows the relationship of the aneurysm with cavernous sinus (3) roof and left clinoid processes (4,5). (C) The arterial model shows an SCA aneurysm (6) involving the origin of the left posterior cerebral artery and the left superior cerebellar artery (7) and related to the P1 (7) and P2 (8) segments.
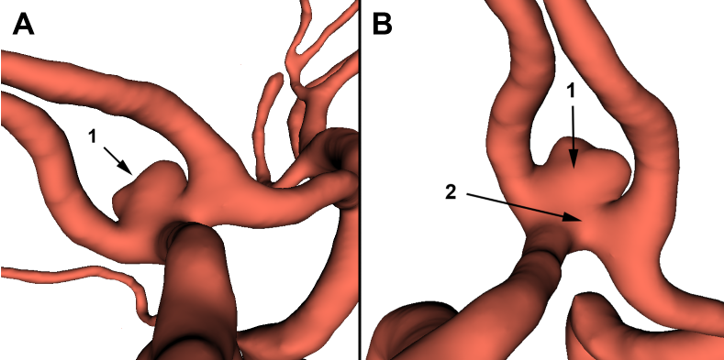
Figure 6.3D slicer modeling of the anterior communicating artery complex: An aneurysm (1) involves the anterior communicating artery (2).
Discussion
Paraclinoid aneurysms
Paraclinoid aneurysms involve the ophthalmic segment of the internal carotid artery (ICA), extending between the dural ring and the PCOA [7,8]. This type accounts for 5-15% of all intracranial aneurysms. De Oliveira classified paraclinoid aneurysms into superior and inferior types and considered medial and lateral ones as an expansion of these types. Even if this classification is simple, it does not accurately reflect the exposure of the aneurysm and related technical difficulties. So, Krisht and Hsu proposed four morphological types. The superior type comprises ophthalmic and dorsal ICA aneurysms, the medial type includes superior hypophyseal and carotid-cave aneurysms, and the lateral develops within the anterior clinoid process. All the remaining forms are of the inferior type. This classification is more adapted to surgical considerations [8]. The best treatment modality is still the surgery, even if it is challenging because of the complexity of the paraclinoid region. Luzzi et al. and Bae et al. reported in their studies on paraclinoid aneurysms that the most frequent surgical outcomes were vasospasm and neurological disorders. However, they did not notice rebleeding episodes [7,8].
In our case, the paraclinoid aneurysm was of the inferior type, according to Krisht and Hsu’s classification. It was wide-necked and close to the left clinoid processes, the left PCOA, and the superior wall of the left cavernous sinus. Given these features, muscle wrapping was the best option. The anatomical relationships were visible in the CTA. However, it was presented in 2D plans and did not give an exact vision of the surgical field. An automatic 3D reconstruction was performed. Compared to the 3D slicer model, its quality was clearly inferior and was presented in 2D flat images. However, the 3D slicer modeling gave a high-quality stereoscopic representation of the surgical anatomy, reproducing the surgical observation. Such a realistic simulation could help surgeons choose the best approach and therapeutic option before surgery. Indeed, if surgeons had the 3D slicer model before the surgery, they would have certainly chosen the wrapping option rather than clipping. The literature reports that 3D slicer modeling results agree with intraoperative conditions. It generates a « déjà vu » feeling that enhances surgical confidence [3].
Superior cerebellar artery aneurysms
SCA aneurysms involve the SCA origin or the part of the BA between SCA and PCA origins [9] and represent 1-1.8% of all intracranial aneurysms [5,10,11]. SCA aneurysms may provoke oculomotor palsy because of the close relationship between the SCA and the III cranial nerve [10]. This clinical expression occurs in 3% of cases [5]. For several reasons, surgical management of SCA aneurysm is challenging. The surgeon needs destructive approaches to access it. Since the region harbors perforators and cranial nerves that may be injured, clipping an SCA aneurysm is dangerous. In addition, most surgeons do not have enough experience with SCA aneurysms because of their rarity [5]. However, SCA aneurysms still have better outcomes after microsurgery than other BA aneurysms, with a mortality rate of 4.8%, often because of the severity of the SAH [11].
Posterior cerebral artery aneurysms
PCA aneurysm is rare, with less than 2% incidence. It is often dissecting, has different shapes, rarely saccular. It often involves P1 and P2, rather than P3 and P4 segments. PCA aneurysm surgery is also challenging because of anatomical relationships with surrounding perforators, deep veins, and cranial nerves [4]. In our case, the CTA with 3D mode showed that the posterior aneurysm involved the left PCA. However, 3D slicer modeling showed an SCA aneurysm incorporating the origin of the PCA. The results met surgical findings again. This discordance with the radiologist’s interpretation was related to the 2D aspect of the CTA. Indeed, the original stem of the SCA and the aneurysm overlapped in the three views of the CTA, and the radiologist could not discern the origin of the SCA. Given the small caliber of the left SCA, its origin was not clearly visible in the 3D CTA. In the 3D slicer modeling, the manual drawing allows us to model small-caliber vessels. Then, with the rotation tool, we create an appropriate angle of view to discern the SCA origin. Reviewing the literature, we found only two cases of aneurysms involving SCA and PCA origins reported by Aguilar-Perez et al. and Signorelli et al. In both cases, the aneurysm neck was not clipped but reconstructed by pCONus-assisted coiling [12]. In our case, the aneurysm neck presented a safety margin to isolate SCA and PCA origins from the clipping zone. So, it was possible to clip it.
The incidental diagnosis of the ACOA aneurysm
The 3D slicer modeling incidentally revealed an ACOA aneurysm. When we revised the CTA and 3D CTA images, a little outpouching from the ACOA drew our attention (Figure 4, Figure 7, Figure 8). Regarding the anatomical layout of the ACOA complex, it was difficult to determine whether the lesion was an aneurysm or to decide on its origin. Given the urgency and within the limits of the CTA images, we believe that the radiologist and neurosurgeons did not analyze the ACOA complex properly. It is probably why they missed the diagnosis. In the 3D slicer modeling, we used an uncommon view angle to see the aneurysm. In addition, with the automatic segmentation, we got a good-quality 3D model in a few minutes. Thus, 3D slicer modeling is adaptable to urgency. Neurosurgeons were surprised by the quality of the 3D model since it met the surgical findings and added new features about the ACOA aneurysm. They believed that, with this method, they would have optimized the therapeutic choice and avoided the tragedy. Indeed, if 3D modeling had been done before surgery, the ACOA aneurysm would have been diagnosed and treated. The patient would have less risk of rebleeding.
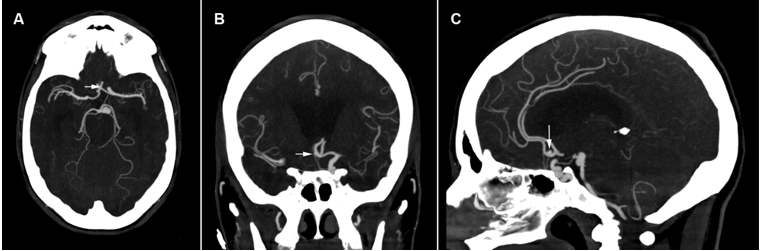
Figure 7.Sagittal, axial, and coronal brain CTA images: the white arrow indicates a suspicious lesion of the anterior communicating artery.
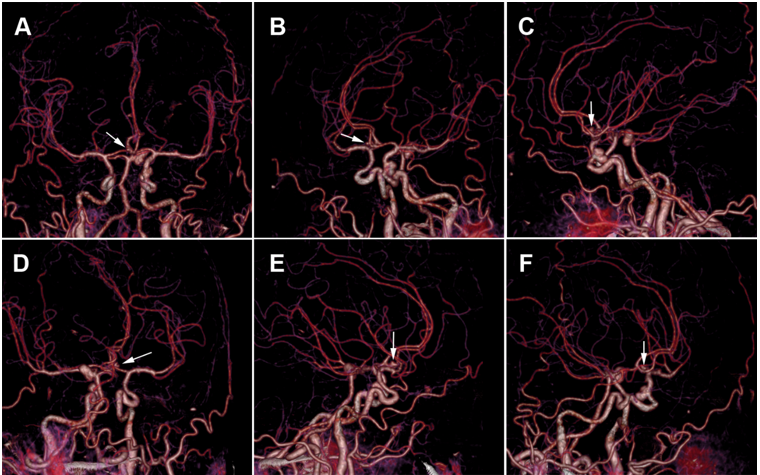
Figure 8.3D brain CTA images: the white arrow indicates a suspicious outpouching from the anterior communicating artery.
Comparison between imaging modalities
The DSA could help to diagnose the aneurysm. However, it was unavailable in our community. In addition, DSA has some limits. With unilateral contrast injection, only one-sided A1 and A2 segments are visualized, and the skull is not represented [3]. In addition, DSA remains expansive and invasive [3] and provokes procedure-related complications. It includes ischemia, bleeding, radiation side effects, contrast-induced allergy, and neurological complications [3,6]. 3D-CTA simplifies the vascular representation but sometimes with insufficient size and quality. However, 3D slicer modeling offers a 3D model as the 3D DSA with additional options, such as viewing angle control, image contrast, magnification, and measurement. Especially with the virtual reality option, 3D slicer modeling combines the 3D-DSA precision level to an exact spatial representation of aneurysm relationships with the parent artery and the skull. With such options, the surgeon can optimize the choice of head position, surgical approach, and craniotomy extent. As a result, the neurosurgeon can adapt the exposure of the lesion and surrounding structures and reduce cerebral retraction [3]. 3D Slicer facilitates the manipulation of medical images and enhances the quality of analysis. An open-source version of this software is available. Then, it can run on a computer with ordinary configuration, either at home or in the operating room. In addition, it offers the possibility to segment the images, visualize and register the 3D model, becoming analyzable every time [13]. Finally, 3D slicer modeling is easier, low-cost, less time-consuming, and safer. Unfortunately, the 3D slicer is still not approved by « Food and Drug Administration » for clinical tasks [13] or any regulatory body.
Postprocedural rebleeding
Several studies focused on post-clipping rebleeding risk. First, the International Study of Unruptured Intracranial Aneurysms trials showed no rebleeding at one and four years of follow-up [14,2]. Second, the Barrow Ruptured Aneurysm Trial reported no rebleeding for up to six years [2]. Third, the International Subarachnoid Aneurysm Trial reported a rebleeding rate of 0.93% in the first year and 0.03% in subsequent years. Sorteberg et al. studied 544 patients with aneurysmal SAH, of which 221 underwent surgery, and recorded a postoperative rebleeding rate of 0.9%. They speculated the rebleeding was just an epiphenomenon. They considered frailer aneurysm walls and patients’ poor grades as risk factors [15]. Otherwise, it is still controversial if the wrapping protects from aneurysm re-rupture. Some studies reported a post-wrapping rebleeding rate of 8-17% [16,2]. However, others reported less than 1% [16]. Perrini et al. reported a 20% postoperative rebleeding rate in 15 patients who underwent wrapping and 12% in a systematic review of 197 wrapped aneurysms [17]. No post-wrapping rebleeding occurred in the Safavi-Abbasi et al. series [18,19]. In our case, the rebleeding occurred early. A priori, the time between the surgery and rebleeding was too short to consider a re-rupture of treated aneurysms. Regarding what the literature reports about clipping, we believe the SCA aneurysm was sufficiently secured. However, since the paraclinoid aneurysm was wrapped, a re-rupture was still possible. Probably, the hemorrhage originated from the ACOA and paraclinoid aneurysms.
Conclusion
Aneurysmal subarachnoid hemorrhage is still a world health problem, with high mortality and morbidity stats. Reducing its impact relies on accurate diagnosis and evaluation of the etiology, which determines an optimal management strategy. Despite the radiological exploration progress, there are still cases of misdiagnosis. This paper presents a rare association between a paraclinoid and SCA-PCA aneurysms. It also illustrates a fatal cerebral hemorrhage complicating the rupture of an undiagnosed ACOA aneurysm. This tragic event was related to a lack of diagnosis accuracy related to the radiological tool and the human factor. Although the 3D slicer modeling was retrospective, it highlights the usefulness of this tool. We recommend approving this tool worldwide and using it for aneurysm diagnosis.
Consent for publication: As this work was retrospective and images anonymized, the consent was waived.
Ethical approval: This case report has been performed in accordance with the ethical standards as laid down in the Declaration of Helsinki and the ethical standards of the ethical and deontological committee of the University of Oran 1 Ahmed Ben Bella
Availability of data and material: All data generated or analyzed during this study are included in this published article.
Competing interests: Authors have declared that no competing interests exist
Funding: No funding has been received by the author for preparing this work
References
- Petridis AK, Kamp MA, Cornelius JF, Beez T, Beseoglu K, et al. (2017) Aneurysmal subarachnoid hemorrhage: diagnosis and treatment. Dtsch Arztebl Int. 114(13): 226-236. [PubMed.]
- Toth G, Cerejo R. (2018) Intracranial aneurysms: Review of current science and management. Vasc Med. 23(3): 276-288. [PubMed.]
- Alsofy SZ, Sakellaropoulou I, Nakamura M, Ewelt C, Salma A, et al. (2020) Impact of virtual reality in arterial anatomy detection and surgical planning in patients with unruptured anterior communicating artery aneurysms. Brain Sci. 10(12): 1-16. [PubMed.]
- Hou K, Lv X, Yu J. (2022) Endovascular treatment of posterior cerebral artery trunk aneurysm: the status quo and dilemma. Front Neurol. 12(746525): 1-14. [Ref.]
- Peluso JP, Van Rooij WJ, Sluzewski M, Beute GN. (2007) Superior cerebellar artery aneurysms: incidence, clinical presentation and midterm outcome of endovascular treatment. Neuroradiol. 49(9): 747-751. [PubMed.]
- Nam HH, Jang DK, Cho BR. (2022) Complications and risk factors after digital subtraction angiography: 1-year single-center study. J Cerebrovasc Endovasc Neurosurg. 24(4): 335-340. [PubMed.]
- Bae DH, Kim JM, Won YD, Choi KS, Cheong JH, et al. (2014) Clinical outcome of paraclinoid internal carotid artery aneurysms after microsurgical neck clipping in comparison with endovascular embolization. J Cerebrovasc. Endovasc. Neurosurg. 16(3): 225-234. [PubMed.]
- Luzzi S, Lucifero AG, Baldoncini M, Del Maestro M, Elbabaa SK, et al. (2022) Paraclinoid aneurysms: outcome analysis and technical remarks of a microsurgical series. Interdiscip Neurosurg Advanc Tech. 27: 1-16. [Ref.]
- Lemos-Rodríguez AM, Sreenath S, Unnithan A, Doan V, Recinos PF, et al. (2016) A New window for the treatment of posterior cerebral artery, superior cerebellar artery, and basilar Apex aneurysm: the expanded endoscopic endonasal approach. J Neurol Surg B Skull base. 77(4): 308-313. [Ref.]
- Nair P, Panikar D, Nair A, Sundar S, Ayiramuthu P, et al. (2015) Microsurgical management of aneurysms of the superior cerebellar artery-lessons learnt: an experience of 14 consecutive cases and review of the literature. Asian J Neurosurg. 10(1): 47-48. [PubMed.]
- Rodríguez-Hernández A, Walcott BP, Birk H, Lawton MT. (2017) The superior cerebellar artery aneurysm: a posterior circulation aneurysm with favorable microsurgical outcomes. Neurosurg. 80(6): 908-916. [PubMed.]
- Signorelli F, Sturiale CL, La Rocca G, Albanese A, D’Argento F, et al. (2017) Giant basilar artery aneurysm involving the origin of bilateral posterior cerebral and superior cerebellar arteries: neck reconstruction with pCONus-Assisted coiling. In: Trends in Reconstructive Neurosurgery. Acta Neurochirurgica Supplement, edited by Visocchi M., Mehdorn HM, Katayama Y, von Wild KRH. 129-134. [PubMed.]
- Wang HW, Wu C, Xue Z, Shu XJ, Sun ZH. (2021) A supplemental technique for preoperative evaluation of giant intracranial aneurysm. J Neurol Surg A Cent Eur Neurosurg. 82(5): 424-429. [PubMed.]
- Schartz D, Mattingly TK, Rahmani R, Ellens N, Akkipeddi SMK, et al. (2021) Noncurative microsurgery for cerebral aneurysms: a systematic review and meta-analysis of wrapping, residual, and recurrence rates. J Neurosurg. 137(1-11): 129-139. [PubMed.]
- Sorteberg A, Romundstad L, Sorteberg W. (2021) Timelines and rebleeds in patients admitted into neurosurgical care for aneurysmal subarachnoid haemorrhage. Acta Neurochir. 163(3): 771-781. [Ref.]
- Baldoncini M, Wahjoepramono EJ, Wahjoepramono PO, Campero A, Justa AF, et al. (2021) Wrapping technique in fusiform aneurysms. Neurol Sci Neurosurg. 2(1): 1-6. [Ref.]
- Perrini P, Montemurro N, Caniglia M, Lazzarotti G, Benedetto N. (2015) Wrapping of intracranial aneurysms: single-center series and systematic review of the literature. Br J Neurosurg. 29(6): 785-791. [PubMed.]
- Safavi-Abbasi S, Kalani MYS, Frock B, Hai Sun B, Yugmurlu K, et al. (2017) Techniques and outcomes of microsurgical management of ruptured and unruptured fusiform cerebral aneurysms. J Neurosurg. 127: 1353-1360. [PubMed.]
- Safavi-Abbasi S, Moron F, Sun H, Wilson C, Frock B, et al. (2016) Techniques and outcomes of Gore-Tex clip-wrapping of ruptured and unruptured cerebral aneurysms. World Neurosurg. 90: 281-290. [PubMed.]