>Corresponding Author : Mehdi Belhakim
>Article Type : Case Report
>Volume : 3 | Issue : 9
>Received Date : 02 Dec, 2023
>Accepted Date : 15 Dec, 2023
>Published Date : 20 Dec, 2023
>DOI : https://doi.org/10.54289/JCRMH2300144
>Citation : Belhakim M, M'chanter H, Aslaoui J and Habbal R. (2023) Neoplastic Pericardial Effusion in Malignant Solid Tumors: Clinical Features, Diagnosis and Management. J Case Rep Med Hist 3(9): doi https://doi.org/10.54289/JCRMH2300144
>Copyright : © 2023 Belhakim M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access
1Cardiology department, University Hospital Ibn Rochd, University Hassan II, Casablanca, Morocco
2Oncology departement, University Hospital Ibn Rochd, University Hassan II, Casablanca, Morocco
*Corresponding author: Mehdi Belhakim, Cardiology department, University Hospital Ibn Rochd, University Hassan II, Casablanca, Morocco
Abstract
Introduction: Neoplastic pericardial effusion (NPE) poses a significant challenge in medical pathology, particularly due to its association with secondary involvement by malignancies originating in various organs. Timely management of NPE is pivotal for both overall survival and the patients' quality of life. However, there is a scarcity of data on NPE in our country. This study aims to provide a comprehensive description of the clinical, diagnostic, and therapeutic characteristics of consecutive Moroccan patients with NPE.
Methods: A retrospective cohort study was conducted at Ibn Rochd Cardiology Department and Oncology Unit in Casablanca, covering a period of three years (January 2020 to December 2022). The study included patients diagnosed with NPE based on clinical, biological, histological, and echocardiographic criteria. Data collection encompassed sociodemographic information, performance status, clinical manifestations, ECG and echocardiography findings, grade of pericardial effusion, cytology of pericardial fluid, and details of therapeutic protocols administered.
Results: Between 2020 and 2022, 49 NPE patients were admitted, with a gender distribution of 51% men and 49% women. The median age was 42 years. Most patients presented with symptomatic pericardial effusion (81%), and 61.2% had NPE at the first diagnosis of metastatic disease. Lung, breast, and other solid tumors were common etiologies. Cytology of pericardial fluid was positive in 23 patients. Clinical examination and diagnostic imaging revealed diverse presentations, including signs of right heart failure, ECG abnormalities, and echocardiographic findings indicative of significant pericardial effusion. Pericardiocentesis and drainage were performed in the majority of patients, often combined with pleuropericardial window placement. Systemic treatments, tailored to the primary tumor type, were administered, with a 60% overall mortality rate, rising to 88% in lung cancer cases.
Conclusion: This study provides crucial insights into the clinical spectrum and management of NPE in Moroccan patients. The high mortality rate, particularly in lung cancers, underscores the importance of early detection and targeted therapeutic interventions in improving outcomes for patients with NPE.
Keywords: Neoplastic Pericardial Effusion; Solid Malignant Tumors; Cardiology; Oncology, Morocco
Abbreviations: PE: Pericardial Effusion, NPE: Neoplastic Pericardial Effusion, PS: Performance Status, ECG: Electrocardiogram, TTE: Transthoracic Echocardiography, UHC: University Hospital Center, NSCLC: Non-Small Cell Lung Cancer, SCLC: Small Cell Lung Cancer, VATS: Video-Assisted Thoracic Surgery
Introduction
Neoplastic pericardial effusion (NPE) represents a pervasive and consequential issue within the realm of medical pathology. While primary pericardial tumors are rare, secondary involvement by neoplasms originating in other organs such as lung and breast is more prevalent [1,2]. Promptly managing NPE is crucial for both overall survival and the quality of life of patients [3,4]. However, few data are available on NPE in our country. The aim of this study is to describe clinical, diagnostic, and therapeutic attributes in a contemporary cohort of consecutive Moroccan patients with NPE.
Materials and Methods
Study Design, Location, and Duration
A retrospective cohort of patients, who were diagnosed with NPE in Ibn Rochd Cardiology Departement in Casablanca, was selected. The study period encompassed a span of 3 years, from January 2020 to December 2022.
Study Population
Inclusion Criteria: This study included patients under the care of the Cardiology Department and Oncology Unit in Ibn Rochd Hospital in Casablanca, whom were diagnosed with pericardial effusionof solid malignant tumors based on a combination of clinical, biological, histological and echocardiographic criterias.
Exclusion Criteria: Patients whose diagnosis of malignant pericarditis did not meet international diagnostic criteria and those in whom pericardial effusion was not directly attributed to neoplastic pathology were excluded from the analysis. Patients diagnosed with PE due to malignant hemopathies were also excluded.
Data Collection: The Data were retrospectively recovered from the medical records of patients admitted to the Cardiology Department andOncology Unit in Ibn Rochd Hospital in Casablanca during the specified timeframe using a structured data collection grid. The collected data encompassed the following categories:
• Sociodemographic information (age, gender)
• Performance status (PS)
• Clinical manifestations (asymptomatic, symptomatic, dyspnea or cardiac tamponade).
• Physical examination findings.
• Electrocardiogram (ECG) and cardiac Doppler echocardiography findings.
• Grade of PE:mild, moderate or severe.
• Cytology of PE: positive or negative.
• Timing of presentation:first diagnosis of metastatic disease or subsequent disease progression.
• Details of various therapeutic protocols administered.
• Follow-up duration.
Results
From 2020 to 2022, 49 patients with neoplastic pericardial effusion (NPE) were admitted to our institution. Twenty-fivewere men (51%) and 24 were women (49%). The median age was 42 years (19–75). The majority of patients had PS≥2 (65%) and presented symptomatic Pericardial effusion (81%): dyspnea in 37 patients (57.5%), cardiac tamponade in 10 patients (20%). NPE occurred in 21 patients with lung, 19 with breast, and 9 with other solid tumors. Pericardial effusion (Figure 1) was detected at first diagnosis of metastatic disease in 30 patients (61.2%). In six patients (12.2%), pericardium was the only site of distant metastases. All patients had cytology of pericardial fluid, which was positive in 23 patients. Among lung tumors, non-small cell lung cancer (NSCLC) was the most frequent histology (10 adenocarcinoma and 5 squamous cell carcinoma). Small cell lung cancer (SCLC) was diagnosed in6 patients. Most of the lung cancer patients had PS≥2(62%), symptomatic presentation (66%), concomitant extrapericardial metastases (71.4%), severe PE (62%), and presented NPE at first diagnosis (47%).Clinical examination revealed signs of right heart failure in 38.7% of cases, muffled heart sounds (67.3%), arterial hypotension (16.3%), and tachycardia (63.2%). ECG showed sinus tachycardia in 63.2% of cases, low voltage(57.1%) (Figure 2) , and electrical alternans in 16.3%.Transthoracic echocardiography revealed significant pericardial effusion in 79.5% of cases, significant mitral or tricuspid flow variation in 24.4%, compression of right heart chambers in 18.3%, and inferior vena cava dilation in 65.3% of cases. The fluid appeared serohemorrhagic in 75.5% of cases and citrine yellow in 24.5% of patients, with an exudative predominance in 87.7%.
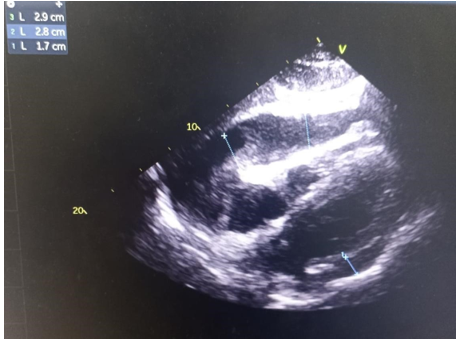
Figure 1. Neoplastic Pericardial effusion with secondary right ventriculaire compression.
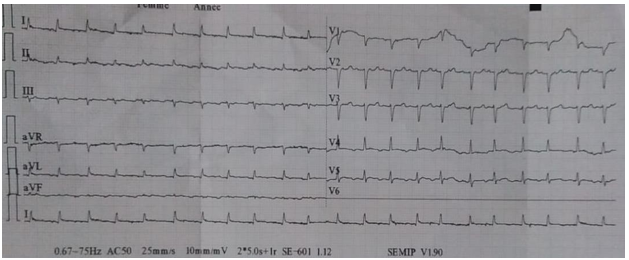
Figure 2. Sinus tachycardia with a low voltage on ECG.
All of our patients underwent pericardiocentesis, with the majority undergoing pericardial drainage (83.6%), often combined with a pleuropericardial window in 61% of cases. Most patients received systemic treatment, including chemotherapy or targeted agents based on their molecular profile.
Systemic treatment for NEP of pulmonary origin included: adenocarcinoma (Vinorelbine cisplatin in 3 patients, pemetrexed cisplatin in 6 patients, and anti-ALK therapy in 1 patient); squamous cell carcinoma (vinorelbine cisplatin in 5 patients and immunotherapy in 2 patients); small cell carcinoma (etoposide cisplatin in 5 patients and immunotherapy in 1 patient). Systemic treatment for breast cancer-related neoplastic pericarditis involved sequential chemotherapy with anthracyclines and taxanes in 5 patients with a triple-negative molecular profile, targeted anti-HER2 therapy in 4 patients with an HER2 molecular profile, and hormone therapy in 10 patients with hormone receptor-positive tumors. Two cases of thymoma were treated with local radiation therapy at a dose of 50 Gy.
As of the latest update, the overall mortality rate among our patients was 60%, with a mortality rate of 88% in lung cancers.
Discussion
Pericardial effusion (PE) represents a perilous complication arising in the context of malignancies and is intricately linked to an unfavorable prognostic outcome. Our study has enabled us to evaluate the diagnostic and therapeutic approaches to Moroccan patients with NEP over the past 3 years within our hospital setting. This evaluation had unveiled the manifestation of lung cancer in 42.8% of the patients succeeded by breast cancer in 38.7%. These findings were congruent with two retrospective series [5,6].
Pericarditis denotes an inflammatory condition affecting the pericardium, typically manifesting with an acute and abrupt onset.
Clinical manifestations encompass tachycardia, thoracic discomfort, and the detection of a pericardial friction rub [7]. The presence of pericardial effusion is variable, often exhibiting subtlety. Certain individuals may remain asymptomatic, whereas others may experience dyspnea, fatigue, or palpitations attributable to cardiac compression and constraints on cardiac filling. Additionally, a subset of patients may manifest non-specific or atypical symptoms such as nausea, dysphagia, hoarseness, and hiccups, arising from the compression of adjacent anatomical structures [8]. The clinical presentation of effusion correlates with the rapidity of its onset. Individuals afflicted with pericarditis commonly exhibit saddle-shaped or concave ST segment elevation, coupled with PR depression across the majority of limb leads (I, II, III, aVL, aVF) and precordial leads (V2V6). Concomitant with the pain experienced, sinus tachycardia frequently manifests in patients with acute pericarditis. The absence of reciprocal ST segment alterations in acute pericarditis serves as a valuable discriminant from myocardial infarction [9]. In individuals affected by pericardial effusion, electrocardiograms (ECGs) often reveal PR segment depression and diminished QRS voltage [10,11]. The presence of a substantial pericardial effusion may be characterized by a triad of low QRS voltage, sinus tachycardia, and electrical alternans. The principal diagnostic modality for establishing pericardial effusion is transthoracic echocardiography. This imaging technique not only aids in diagnosis but is also instrumental in quantifying the hemodynamic impact of the effusion on the heart and determining the urgency of intervention. In cases where immediate intervention is unnecessary, echocardiographic imaging remains the primary tool for continuous monitoring and reassessment of changes in pericardial effusion. The echocardiographic assessment should encompass the identification and loculation of the effusion, determination of size, evaluation of fluid characteristics, and assessment of hemodynamic compromise (40). In adults, the classification of pericardial effusion at end-diastole considers it small if the distance between the visceral and parietal pericardium is < 10 mm, moderate if between 10 and 20 mm, and large if > 20 mm [12,13]. Hemodynamic effects are predominantly observed on the right side [13,14]. Right atrium wall compression and collapse during ventricular systole is an early indication of tamponade; right ventricular collapse in early diastole is even more specific; left atrial and ventricular collapse are less common as they are a late finding and require emergent intervention [15]. Fluid cytology has a high specificity but low sensitivity [16]. The consideration of pericardial disease associated with malignancy should not be dismissed solely based on negative cytology results from pericardiocentesis. Absence of malignant cells in cytological analysis is observed in approximately two-thirds of patients with coexisting pericardial effusion and malignancy, potentially necessitating further biopsy. Notably, the presence of positive cytology has been correlated with poorer outcomes in individuals with a pre-existing malignancy [16]. While patients displaying minimal or no symptoms may be subject to observation without immediate intervention, those experiencing pronounced symptoms or hemodynamic compromise as a result of cardiac tamponade necessitate prompt pericardiocentesis [17]. This approach addresses the urgency of the situation and affords time for strategic planning of definitive management [16]. It is crucial to note that isolated pericardiocentesis is linked to recurrence rates ranging between 33% and 62% [18,19]. In the contemporary era, the utilization of routine echocardiographic guidance has contributed to a notable reduction in complication rates associated with the procedure. Pericardiotomy involves the excision of a segment of pericardial tissue. This procedure facilitates the drainage of pericardial fluid into either the pleural space or subcutaneous tissues, where the fluid can be more effectively absorbed. The creation of a pericardial window, commonly performed through either the subxiphoid or video-assisted thoracic surgery (VATS) approach, involves making an incision below the xiphoid process in the subxiphoid method. Dissection is carried out to the anterior surface of the pericardium behind the sternum, often necessitating the removal of the xiphoid process. A piece of pericardial tissue is excised, allowing for fluid drainage and potential biopsy. The subxiphoid approach, often done under local anesthesia, presents advantages, including a low recurrence rate and reduced risk of hemodynamic decompensation during induction of general anesthesia in cases of early tamponade physiology. Careful confirmation of effusion accessibility is crucial to prevent complications, such as cardiac injury [16].
The prescription of systemic treatment was carried out in eligible patients following international recommendations [20,21]. Locoregional radiotherapy was performed in 2 patients with thymoma and 3 patients with superior vena cava syndrome. The outcome was marked by the death of more than half of our patients, which could be explained by the frequency of multi-metastatic forms in our series, consistent with literature data. Elisabetta Di Liso et al. reported a death rate of 89% in 29 patients with neoplastic pericarditis [6]. In a case series published by Rhesma S et al., all patients had died at the time of the report [22].
Conclusion
In conclusion, our study sheds light on the diagnostic and therapeutic landscape of Neoplastic Pericardial Effusion (NPE) in a Moroccan cohort over a three-year period. The prevalence of lung and breast cancers as primary contributors to NPE aligns with existing literature. The high mortality rate, particularly in lung cancer cases, underscores the critical need for early detection and targeted interventions. Our findings emphasize the importance of a multidisciplinary approach, incorporating clinical, imaging, and therapeutic modalities, to enhance outcomes in patients grappling with this challenging condition.
Declarations
Ethics approval and consent to participate: This study was in accordance with the tenants of the Declaration of Helsinki. Ethical approval was not required for this retrospective study at our institution.
Consent for publication: Written informed consent was obtained from the patient for publication and any accompanying images. A copy of the written consent is available for review by the Editor-inChief of this journal on request.
Availability of data and material: The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Competing interests: The authors declare that they have no competing interests.
Fundings: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contributions: All authors contributed to the study conception and design and commented on previous versions of the manuscript.
All authors read and approved the final manuscript.
Acknowledgment: Not applicable
References
- Refaat MM, Katz WE. (2011) Neoplastic Pericardial Effusion. Clin Cardiol. 34(10): 593‑598. [Ref.]
- Retter AS. (2002) Pericardial disease in the oncology patient. Heart disease (Hagerstown, Md). 4(6): 387‑391. [PubMed.]
- Burazor I, Imazio M, Markel G, Adler Y. (2013) Malignant pericardial effusion. Cardiology. 124(4): 224‑232. [PubMed.]
- Pawlak A, Szturmowicz M, Fija lkowska A, Gątarek J, Gralec R, et al. (2012) Diagnosis of malignant pericarditis: a single centre experience. Kardiologia Polska (Polish Heart Journal). 70(11): 1147‑1153. [PubMed.]
- Abraham KP, Reddy V, Gattuso P. (1990) Neoplasms metastatic to the heart: review of 3314 consecutive autopsies. Am J Cardiovasc Pathol. 3(3): 195‑198. [PubMed.]
- Di Liso E, Menichetti A, Dieci MV, Ghiotto C, Banzato A, et al. (2019) Neoplastic Pericardial Effusion: A Monocentric Retrospective Study. Journal of Palliative Medicine. 22(6): 691‑695. [PubMed.]
- Pawlak Cieslik A, Szturmowicz M, Fijalkowska A, Gatarek J, Gralec R, et al. (2012) Diagnosis of malignant pericarditis: a single centre experience. Kardiol Pol. 70(11): 1147-1153. [PubMed.]
- Imazio M, Adler Y. (2013) Management of pericardial effusion. Eur Heart J. 34(16): 1186-1197. [Ref.]
- Marinella MA. (1998) Electrocardiographic manifestations and differential diagnosis of acute pericarditis. Am Fam Physician. 57(4): 699-704. [PubMed.]
- Eisenberg MJ, de Romeral LM, Heidenreich PA, Schiller NB, Evans GT Jr. (1996) The diagnosis of pericardial effusion and cardiac tamponade by 12-lead ECG. A technology assessment. Chest. 110(2): 318-324. [PubMed.]
- Kudaiberdiev T, Dzhumagulova A, Joshibayev S, Tilemanbetova K, Imanalieva G. (2016) Electrocardiographic abnormalities in patients with pericardial disease—association of PR segment depression with arrhythmias and clinical signs: experience of cardiac surgery center. J Electrocardiol. 49(1): 29-36. [PubMed.]
- Perez-Casares A, Cesar S, Brunet-Garcia L, Sanchez-de-Toledo J. (2017) Echocardiographic evaluation of pericardial effusion and cardiac tamponade. Front Pediatr. 5: 79. [PubMed.]
- Ceriani E, Cogliati C. (2016) Update on bedside ultrasound diagnosis of pericardial effusion. Intern Emerg Med. 11(3): 477-4780. [PubMed.]
- Vakamudi S, Ho N, Cremer PC. (2017) Pericardial effusions: causes, diagnosis, and management. Prog Cardiovasc Dis. 59(4): 380-388. [PubMed.]
- Schusler R, Meyerson SL. (2018) Pericardial disease associated with malignancy. Current Cardiology Reports. 20: 1-8. [Ref.]
- Gecmen C, Gecmen GG, Ece D, Kahyaoglu M, Kalayci A, et al. (2018) Cytopathology of pericardial effusions: experience from a tertiary center of cardiology. Herz. 43(6): 543-547. [PubMed.]
- Labbe C, Tremblay L, Lacasse Y. (2015) Pericardiocentesis versus pericardiotomy for malignant pericardial effusion: a retrospective comparison. Curr Oncol (Toronto, Ont). 22(6): 412-416. [PubMed.]
- Apodaca-Cruz A, Villarreal-Garza C, Torres-Avila B, Torres J, Meneses A, et al. (2010) Effectiveness and prognosis of initial pericardiocentesis in the primary management ofmalignant pericardial effusion. Interact Cardiovasc Thorac Surg. 11(2): 154-161. [PubMed.]
- Gibbs CR, Watson RD, Singh SP, Lip GY. (2000) Management of pericardial effusion by drainage: a survey of 10 years’ experience in a city centre general hospital serving a multiracial population. Postgrad Med J. 76(902): 809-813. [PubMed.]
- AURA. (2023) Formes métastatiques - cancer-bronchique-non-petites stade IV. [PubMed.]
- ESMO. (2023) Breast Cancer | ESMO Disponible sur breast-cancer. [PubMed.]
- Babu RS, Lanjewar A, Jadhav U, Wagh P, Aurangabadkar G, et al. (2022) A case series of malignant pericardial effusion. J Family Med Prim Care. 11(10): 6581‑6585. [PubMed.]