>Corresponding Author : Mawia Karkoutly
>Article Type : Case Report
>Volume : 4 | Issue : 1
>Received Date : 21 Dec, 2023
>Accepted Date : 05 Jan, 2024
>Published Date : 09 Jan, 2024
>DOI : https://doi.org/10.54289/JCRMH2400102
>Citation : Karkoutly M, Alnassar I, Laflouf M and Bshara N. (2024) Management of Failed Regenerative Endodontic Treatment of a Necrotic Immature Molar: A Case Report With 12-Month Follow Up. J Case Rep Med Hist 4(1): doi https://doi.org/10.54289/JCRMH2400102
>Copyright : © 2024 Karkoutly M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access
Department of Pediatric Dentistry, Damascus University, Syrian Arab Republic
*Corresponding author: Mawia Karkoutly, Department of Pediatric Dentistry, Damascus University, Syrian Arab Republic
Abstract
Introduction: Management of necrotic immature permanent teeth has always posed a challenge to clinicians. Regenerative endodontic procedures (REPs) have been proposed as an alternative to apexification to treat necrotic immature teeth. However, few failed cases of REPs have been presented in the literature with different successful retreatment approaches.
Case presentation: An eight-year-old boy reported spontaneous pain in the right permanent mandibular first molar. Regenerative endodontic treatment using platelet-rich fibrin (PRF) was the treatment method due to the open apices. At the 9-month follow-up, there was a periapical lesion around the distal root. Hence, apexification with Mineral trioxide aggregate (MTA) was carried out. In the twelve-month follow-up, the periapical lesion healed radiographically.
Conclusions: MTA and bioceramic-based root canal sealers yielded satisfactory outcomes in lesion healing. Little is known about the biological and clinical aspects of regenerative endodontic treatment. Moreover, there are still unknown factors that govern the success of REPs.
Keywords: Regenerative Endodontics; Platelet-Rich Fibrin; Mineral Trioxide Aggregate; Bioceramic-Based Root Canal Sealer; Case Report
Abbreviations: MTA: Mineral Trioxide Aggregate, REP: Regenerative Endodontic Procedures, PRF: Platelet Rich Fibrin, ZOE: Zinc Oxide Eugenol, IANB: Inferior Alveolar Nerve Block, WL: Working Length, TAP: Triple Antibiotic Paste, RPM: Revolutions Per Minute, PPP: Platelet Poor Plasma, RBC: Red Blood Cells, AAE: American Association of Endodontists, SCAP: Stem Cells from the Apical Papilla, LPS: Lipopolysaccharides
Background
Management of necrotic immature permanent teeth has always posed a challenge to dental practitioners due to the thin dentin walls, wide open apex, and difficulty cleansing the root canal system of non-vital immature teeth. Furthermore, pulp necrosis can arrest root development and lead to fragile dentin walls, which are more prone to fractures [1]. Therefore, every attempt should be made to maintain the pulp vitality of immature teeth.
Traditionally, apexification with calcium hydroxide was an acceptable approach to inducing a calcified apical barrier in non-vital immature teeth. However, this method has several disadvantages, including multiple visits, long-term treatment, and reinfection possibilities. The previous facts suggested the use of mineral trioxide aggregate (MTA) as an alternative to calcium hydroxide [2,3], which yielded satisfactory outcomes in terms of dentin bridge formation in vital pulp therapy [3] and resolving periapical lesions [4]. Unfortunately, both procedures cannot induce maturation and natural development of the root canal system.
Regenerative endodontic procedures (REPs) have been proposed as a conservative alternative to apexification to treat non-vital immature teeth. REPs aim to thicken and elongate the root canal walls, induce apical closure, promote dentin-pulp complex formation, and restore physiologic functions. Namely, REPs aim to mimic the cellular and molecular mechanisms during tooth maturation. This treatment method has been considered a “paradigm shift” [5]. The three main ingredients for regenerative endodontic treatment are stem cells, growth factors, and scaffolds. Firstly, Stem cells can proliferate and differentiate to induce hard tissue formation. Secondly, growth factors regulate the stimulation of several cellular activities like migration, proliferation, differentiation, and apoptosis. Lastly, scaffolds serve as an extracellular matrix to support tissue ingrowth and provide correct localization for cells and can be either natural or synthetic [5,6]. Platelet-rich fibrin (PRF) is a synthetic scaffold of autologous fibrin loaded with platelet cytokines, leukocyte cytokines, and bioactive molecules [6]. It was first proposed in France by Choukroun et al. in 2001 [6,7]. However, few failed cases of REPs have been presented in the literature with different successful retreatment approaches [8-10].
This report presented a case of management of failed regenerative endodontic treatment of a necrotic immature molar using MTA apical plug and bioceramic-based root canal sealer.
Case presentation
An eight-year-old boy was presented to the Department of Pediatric Dentistry, at Damascus University, in August 2021. He was referred to evaluate the right permanent mandibular first molar after incomplete treatment performed by a general dentist. The patient’s parents reported a previous spontaneous pain lasting for hours and aggravated when the patient lay down, for which his dentist had performed an emergency treatment. The patient was with low socioeconomic status. There was no relevant medical history. In clinical assessment, extraoral examination revealed no swelling or facial asymmetry. Intraoral inspection showed a temporary filling of zinc-oxide eugenol (ZOE) cement (Zitetemp, Prevest DenPro®, Lewes, DE, USA) in the right permanent mandibular first molar. In the diagnostic test, the tooth was tender to percussion and palpation. However, the tooth was non-vital since it was unresponsive to different sensitivity tests. The adjacent gingiva showed a healthy probing depth with physiological tooth mobility. Intraoral radiographic examination showed immature roots, wide open apices, thin dentinal wall, periapical radiolucency, and lamina dura widening (Figure 1). According to clinical and radiographic findings, regenerative endodontic treatment using PRF was considered a treatment option. Written informed consent was provided by the parent’s legal guardians.
Ethical approval was obtained from the institutional review board of Damascus University (N 374/2021), and it was conducted according to the Declaration of Helsinki (2013). On the first appointment, an inferior alveolar never block (IANB) was administered using Lidocaine HCL 2% with Epinephrine 1:80,000 (2% Lidocaine HCL Injection, Huons Co., Ltd, Seongnam, Korea) followed by rubber dam isolation (Sanctuary®, Perak, Malaysia). The temporary filling was removed using a 2-mm round bur (Dentsply, Maillefer, Ballaigues, Switzerland) in a high-speed handpiece (NSK PANA AIR, Nakanishi Inc., Tochigi-ken, Japan) with copious irrigation. Three canals were detected (mesiobuccal, mesiolingual, and distal). Working length (WL) was determined using Root ZX electronic apex locator (J. Morita MFG, Kyoto, Japan, third generation) and was confirmed with radiography. Without mechanical instrumentation, the canals were gently irrigated using 20 mL of 1.5% sodium hypochlorite solution (Carmel®; Akka Brothers Co. Carmel Detergent, Damascus, Syria) and then 20 mL of sterile saline solution (SODIUM CHLORIDE 0.9% MIAMED, Miamed Pharmaceutical Industry, Damascus, Syria). The side-vented needle was inserted 1 mm short of the WL during irrigation. Sterile absorbent paper points (Dentsply, Maillefer, Ballaigues, Switzerland) were used to dry the canals. The canals were filled with triple antibiotic paste (TAP) consisting of an equal proportion of ciprofloxacin (Ceproz, ELSaad Pharmaceuticals, Aleppo, Syria), metronidazole (Statizol, ELSaad Pharmaceuticals, Aleppo, Syria), and minocycline (Quatrocin, ALFARES Pharmaceuticals Co., Damascus, Syria) in a concentration of 1mg/mL, mixed with propylene glycol into a creamy paste using lentulo spiral (Dentsply, Maillefer, Ballaigues, Switzerland). The access cavity was sealed with temporary restoration (Cavit, 3M ESPE, St. Paul, MN, USA).
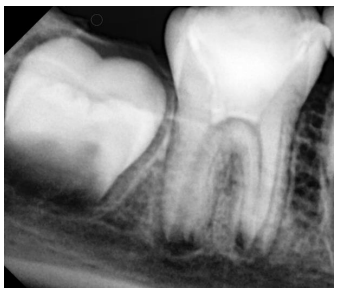
Figure 1. Diagnostic radiograph of the right permanent mandibular first molar showed the presence of periapical radiolucency with lamina dura widening.
The following treatment session was appointed to be 3 weeks later. There was no tenderness to palpation or percussion. An IANB was administered followed by rubber dam isolation. The access cavity was reopened, the intracanal dressing was flushed out of the canals by sterile saline solution irrigation, then the canals were irrigated with 20 mL of 17% EDTA (EDTA Solution, Prevest DenPro®, Lewes, DE, USA). Ultimately, they were rinsed with sterile saline solution. The canals were dried with absorbent paper points. In the meantime, PRF was prepared by drawing a 5 mL sample of whole venous blood from the patient’s right foramen (right median cubital vein). The collected venous blood sample was transferred into a vacutainer tube (Vacuum Blood Collection Red Top Plain Tube, Jiangsu Nuohong Medical Technology Co., Ltd., Anhui, China) without anticoagulant and centrifugated (REMI Laboratories, Mumbai, Maharashtra, India) at 3000 revolutions per minute (rpm) for 10 minutes. Three layers were obtained: an acellular plasma layer (PPP) at the top, PRF in the middle, and a red blood cells layer (RBCs) at the bottom (Figure 2). A sterile tweezer was used to remove the jelly PRF from the vacutainer tube, then it was placed on a dry gauge to squeeze out the fluid present in the fibrin matrix. The freshly prepared PRF was fragmented into small increments and was inserted apically in the root canals up to the middle third and condensed using an endodontic plugger (Elite Dental Products, Daive, Florida, USA). A 2-mm thick layer of white MTA (ProRoot; Dentsply Tulsa Dental Specialty, Tulsa, OK, USA) was placed on the top of the floor of the pulp chamber and then sealed with a wet cotton pellet and temporary filling (Cavit, 3M ESPE, St. Paul, MN, USA) (Figure 3). On the next day, the temporary restoration and the wet cotton pellet were removed, then GIC coronal filling was placed and a stainless steel crown (3M ESPE, St. Paul, MN, USA) was adjusted and cemented with luting glass ionomer cement (GC Fuji I, Leuven, Belgium). At 3- and 6-month follow-ups, the tooth was asymptomatic, with no sensitivity to palpation or sensitivity tests. At the 9- month follow-up, there was a periapical radiolucency around the distal root, tenderness to palpation and percussion, and a negative response to different vitality tests. However, the periapical lesions were resolved around the mesial canals (Figure 4). Therefore, apexification with MTA was considered an optimal retreatment option for the distal canal.

Figure 2. Three layers were obtained after centrifugation: an acellular plasma layer (PPP) at the top, a platelet-rich fibrin layer (PRF) in the middle, and a red blood cells layer (RBCs) at the bottom.
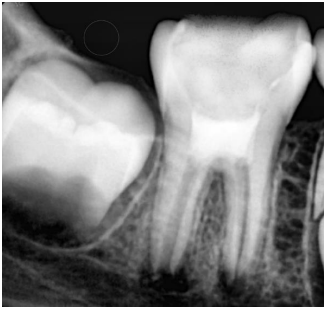
Figure 3. Postoperative radiograph after regenerative treatment and MTA placement.
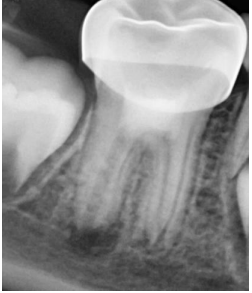
Figure 4. Follow-up radiograph after 9 months showed a periapical lesion around the distal root and bony healing around the mesial roots.
A conventional IANB was administered. After the removal of the stainless steel crown, the tooth was isolated with a rubber dam. The 2 mm thick layer of white MTA was removed with CPR ultrasonic tips (Obtura Spartan Endodontics, Algonquin, IL, USA). After WL determination (Figure 5), the distal root was slightly shaped with stainless steel K-file (Dentsply, Maillefer, Ballaigues, Switzerland), and the mesial roots were prepared using crown down technique. The canals were irrigated using 20 mL of 2.5% sodium hypochlorite solution, followed by 20 mL of sterile saline solution rinsing, then the canals were dried with sterile absorbent paper points. TAP was applied, then the tooth was sealed with a temporary restoration, and the next visit was appointed 21 days later.
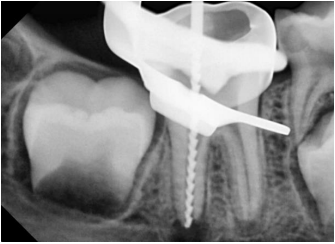
Figure 5. Working length determination in the distal canal
On the next visit appointment, an IANB was administered followed by rubber dam isolation. The access cavity was reopened, and the distal root was rinsed with 20 mL of 2.5% sodium hypochlorite solution, followed by 20 mL of sterile saline solution, and then dried with absorbent paper points. MTA apical plug was applied in small increments. At first, 30 gutta-percha cones (Dentsply, Maillefer, Ballaigues, Switzerland) were used to transfer the MTA increments into the apical third of the distal root, then finally were condensed with the aid of an endodontic plugger into a 5 mm apical plug. A moist cotton pellet was placed over the MTA apical plug, and the tooth was sealed with a temporary restoration.
After 48 hours, an IANB was administered, the tooth was isolated, and the access cavity was reopened. The WL was determined (Figure 6). The canals were rinsed with 20 mL of 2.5% sodium hypochlorite solution, followed by 20 mL of sterile saline solution, and dried with absorbent paper points. The mesial and distal canals were sealed with a bioceramic-based root canal sealer (CeraSeal, Meta Biomed, Chungcheongbuk-do, Korea), then GIC coronal filling was placed on the toot filling materials, and a stainless steel crown was adjusted and cemented with luting glass ionomer cement (Figure 7). In the three-month follow-up, the tooth was asymptomatic, and the periapical lesion began to resolve (Figure 8). In the six-month follow-up, the periapical lesion healed (Figure 9), and in the twelve-month follow-up, the periapical lesion fully healed (Figure 10).
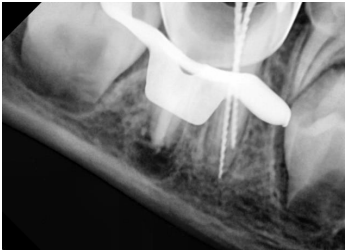
Figure 6. Working length determination in the mesial canals
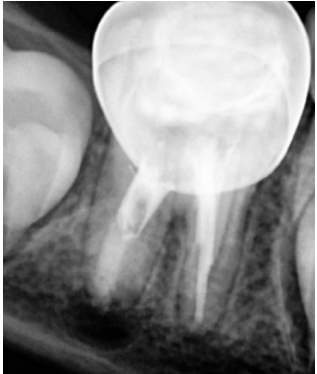
Figure 7. Postoperative radiograph after MTA apical plug placement and sealing with bioceramic-based root canal sealer.
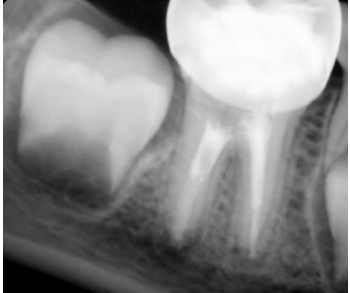
Figure 8. Follow-up radiograph after 3 months, the periapical lesion around the distal root began to resolve.
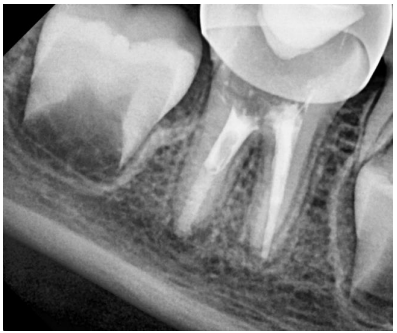
Figure 9. Follow-up radiograph after 6 months, the periapical lesion has resolved.
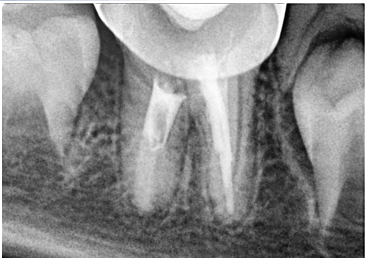
Figure 10. Follow-up radiograph after 12 months, the periapical lesion fully healed.
Discussion
This article presented a case report on a failed regenerative endodontic treatment and its clinical management. Although the success of REPs was highly reported in the literature [11-14], few cases described unfavorable outcomes and their further management [8-10]. The success of REPs is governed by the stage of root maturation [5,15], the size of the apical diameter [5,16,17], the cytotoxicity of the root canal irrigants, the antimicrobial efficacy of the intracanal medicament, and the long-standing nature of the previous infection [5].
According to Cvek et al. [15] classification of the stages of root maturation, REP is suitable for stage 1 (wide divergent apical opening with less than 50% of root length), stage 2 (wide divergent apical opening with 50% of root length), and stage 3 (wide divergent apical opening with 66% of root length). However, for stage 4 (wide open apex with nearly completed root formation), as presented in this case, REP or apexification with MTA apical plug are both suitable treatment options. In addition, Estephan et al. [16] concluded that teeth with a wider diameter (≥1mm) showed better treatment outcomes because this allows the influx of blood vessels and stem cells. The previous facts could explain the failure of the present REP.
Regarding the previous irrigation protocol, the American Association of Endodontists (AAE) recommends using 1.5% sodium hypochlorite solution followed by 17% EDTA [18]. This is according to studies which concluded that sodium hypochlorite is a cytotoxic compound and causes stem cells from the apical papilla (SCAP) damage at concentration greater than 1.5% [19,20]. However, to date, the antimicrobial efficacy of sodium hypochlorite has been extensively tested in vitro experiments [21]. In addition, a reduced concentration of sodium hypochlorite resulted in decreased bactericidal capacity [22]. Therefore, the intracanal antimicrobial capability of 1.5% sodium hypochlorite seems questionable. This could lead to inadequate disinfection, which is the cornerstone for successful REP [5].
In the present case, the previous infection could have damaged the stem cells and the tissue-forming cells in the periapical area resulting in unpredictable revascularization [23]. Pro-inflammatory cytokines (IL-1 and TNF-) can prevent stem cells from differentiating [24-26] despite the immune-regulatory and anti-inflammatory properties of mesenchymal stem cells [27,28] and infection driving mesenchymal stem cells into the site of injury by SDF-1 [29,30]. Furthermore, the presence of lipopolysaccharide shifted SCAP from an odontogenic to an osteogenic phenotype [31].
Mechanical instrumentation was minimal because it could lead to the weakening of the fragile and thin root canal walls [29]. However, the efficacy of mechanical instrumentation protocol in REPs is suspected because it is minimal [5,32]. TAP has been successfully used as an intracanal medicament in REPs due to its antimicrobial efficacy. However, the major drawbacks of TAP is bacterial resistance and coronal discoloration due to minocycline [33].
In this case report, PRF was used as a scaffold because it is rich in growth factors compared with the blood clot scaffold, which could result in favorable treatment outcomes [6]. PRF is widely used in pediatric dentistry since it is a simplified process, and anticoagulants are not required for PRF preparation. It can be used as a scaffold for revascularization in young permanent teeth, pulpotomy medicament, pulp capping material, and surgical wound closure. However, the resulting amount of PRF is low due to the autologous blood. Furthermore, drawing blood from pediatric patients can be challenging due to their lack of compliance [34].
The AAE defines the success of regenerative endodontic treatment by three measures. The primary measure is symptoms resolving and bony healing [18], which is generally achievable [35,36] with high probability (91-94%) [36,37]. The secondary measure is root canal lengthening and/or thickening of the root canal [18], but these outcomes are not always predictable [37-40]. The tertiary goal is a positive response to pulp vitality tests [18], but it does not indicate pulp tissue regeneration [41]. In the present case report, the mesial roots achieved the primary healing measure. This is explained by the fact that the preoperative periapical lesion around the mesial roots is smaller than the one around the distal canal [5].
As mentioned before, the main reason for failed regenerative endodontic cases is inadequate disinfection, inadequate biofilm removal, and the presence of preceding infection, which all lead to root canal reinfection [5].
MTA is highly biocompatible [42,43], has good sealing properties, and has a well marginal adaptation [42,44]. In addition, MTA stimulates the formation of dentin bridges in vital pulp therapy [44] and limits bacterial infection when using it as an apical plug [45]. Furthermore, MTA induces bone deposition [44,46] by stimulating growth factors such as bone morphogenetic protein-2 (BMP-2) and transforming growth factor beta-1 (TGF-β1) to achieve osseous healing [47,48]. Moreover, MTA has yielded satisfactory results in resolving large periapical lesions after six years of follow-up [4]. In the present case report, the periapical lesion around the distal root healed only after six months. This is explained by the well-known fact that regeneration and healing are faster in younger individuals than in older age groups [49,50]. However, the long setting time and the poor handling properties of MTA can be significant shortcomings in pediatric dentistry [51]. Bioceramic-based root canal sealer was used due to its high biocompatibility [52,53], bioactivity [54], and low cytotoxicity [52,53]. Bioceramic sealers have superior properties compared with other sealers in bone deposition and osteogenic potential [54,55]. Moreover, bioceramic sealers stimulate osteogenic differentiation by inhibiting the expression of inflammatory mediators prompted by lipopolysaccharides (LPS), suggesting that these sealers demonstrate anti-inflammatory properties [54].
Conclusion
The results of the present case report suggest that little is known about the biological and clinical aspects of REP, and there are many unanswered questions. Moreover, there are still unknown factors that govern the success of REP. Therefore, further studies should be conducted with a large sample size to decipher this medical mystery. However, this approach is conservative.
Acknowledgements: Not applicable.
Conflict of interest: The authors declare that there are no conflicts of interest.
Funding: The authors declare that they received no external funding to perform the present study.
Ethical approval: Ethical approval was obtained from the institutional review board of Damascus University (N 374/2021). Informed consent from the subject and their legal guardian(s) were obtained for both study participation and publication of information/images in an online open-access publication.
Consent for publication: Informed consent from the subject and their legal guardian(s) were obtained for both study participation and publication of information/images in an online open-access publication.
Data availability statement: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions: M.K. research concept and design, collection and/or assembly of data, data analysis and interpretation, writing the article; I.A. collection and/or assembly of data; M.L. critical revision of the article; N.B. critical revision of the article, final approval of the article.
Preprint: A preprint has previously been published, [56].
References
- Hargreaves KM, Giesler T, Henry M, Wang Y. (2008) Regeneration potential of the young permanent tooth: what does the future hold? Pediatric dentistry. 30(3): 253-260. [PubMed.]
- Rafter M. (2005) Apexification: a review. Dental Traumatology. 21(1): 1-8. [PubMed.]
- Roberts HW, Toth JM, Berzins DW, Charlton DG. (2008) Mineral trioxide aggregate material use in endodontic treatment: a review of the literature. Dental materials. 24(2): 149-164. [PubMed.]
- Yildirim T, Gencoglu N. (2010) Use of mineral trioxide aggregate in the treatment of large periapical lesions: reports of three cases. European Journal of Dentistry. 4(04): 468-474. [Ref.]
- Kim SG, Malek M, Sigurdsson A, Lin LM, Kahler B. (2018) Regenerative endodontics: a comprehensive review. International endodontic journal. 51(12): 1367-1388. [PubMed.]
- Gathani KM, Raghavendra SS. (2016) Scaffolds in regenerative endodontics: A review. Dental research journal. 13(5): 379-386. [Ref.]
- Choukroun J, Adda F, Schoeffler C, Vervelle A. (2001) Une opportunité en paro-implantologie: le PRF. Implantologie. 42: 55-62. [Ref.]
- Žižka R, Šedý J, Voborná I. (2018) Retreatment of failed revascularization/revitalization of immature permanent tooth–A case report. Journal of clinical and experimental dentistry. 10(2): e185-188. [Ref.]
- Dhaimy S, Dhoum S, Amarir H, El Merini H, Nadifi S, Ouazzani AE. (2017) Pulpo-periodontal regeneration: management of partial failure revascularization. Case Reports in Dentistry. 2017: 8302039. [PubMed.]
- Al-Tammami MF, Al-Nazhan SA. (2017) Retreatment of failed regenerative endodontic of orthodontically treated immature permanent maxillary central incisor: a case report. Restorative dentistry & endodontics. 42(1): 65-71. [Ref.]
- Ramezani M, Sanaei‐rad P, Hajihassani N. (2020) Revascularization and vital pulp therapy in immature molars with necrotic pulp and irreversible pulpitis: A case report with two‐year follow‐up. Clinical case reports. 8(1): 206-210. [PubMed.]
- Pace R, Giuliani V, Di Nasso L, Pagavino G, Franceschi D, Franchi L. (2021) Regenerative Endodontic Therapy using a New Antibacterial Root Canal Cleanser in necrotic immature permanent teeth: Report of two cases treated in a single appointment. Clinical Case Reports. 9(4): 1870-1875. [PubMed.]
- Hosseini S, Chitsaz N, Hamrah MH, Maleki D, Taghizadeh E. (2023) Regenerative Endodontic Management of an Immature Necrotic Premolar Using Advanced Platelet-Rich Fibrin. Case Reports in Dentistry. 2023. [Ref.]
- Kumar JK, Surendranath P, Eswaramoorthy R. (2023) Regeneration of immature incisor using platelet rich fibrin: report of a novel clinical application. BMC Oral Health. 23(1): 1-7. [Ref.]
- Cvek M. (1992) Prognosis of luxated non‐vital maxillary incisors treated with calcium hydroxide and filled with gutta‐percha. A retrospective clinical study. Dental Traumatology. 8(2): 45-55. [PubMed.]
- Estefan BS, El Batouty KM, Nagy MM, Diogenes A. (2016) Influence of age and apical diameter on the success of endodontic regeneration procedures. Journal of endodontics. 42(11): 1620-1625. [PubMed.]
- Fang Y, Wang X, Zhu J, Su C, Yang Y, Meng L. (2018) Influence of apical diameter on the outcome of regenerative endodontic treatment in teeth with pulp necrosis: a review. Journal of Endodontics. 44(3): 414-131. [PubMed.]
- American Association of Endodontists. (2016) Clinical Considerations for a Regenerative Procedure. Revised. [Ref.]
- Trevino EG, Patwardhan AN, Henry MA, Perry G, Dybdal-Hargreaves N, Hargreaves KM, et al. (2011) Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. Journal of endodontics. 37(8): 1109-1115. [PubMed.]
- Martin DE, De Almeida JF, Henry MA, Khaing ZZ, Schmidt CE, Teixeira FB, et al. (2014) Concentration-dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. Journal of endodontics. 40(1): 51-55. [PubMed.]
- Iqbal A. (2012) Antimicrobial irrigants in the endodontic therapy. International journal of health sciences. 6(2). [PubMed.]
- Ayhan H, Sultan N, Cirak M, Ruhi MZ, Bodur H. (1999) Antimicrobial effects of various endodontic irrigants on selected microorganisms. International endodontic journal. 32(2): 99-102. [PubMed.]
- Kim SG. (2016) Infection and pulp regeneration. Dentistry journal. 4(1): 4. [Ref.]
- Lacey DC, Simmons PJ, Graves SE, Hamilton JA. (2009) Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthritis and Cartilage. 17: 735-742. [Ref.]
- Liu C, Xiong H, Chen K, Huang Y, Huang Y, Yin X. (2016) Long‐term exposure to pro‐inflammatory cytokines inhibits the osteogenic/dentinogenic differentiation of stem cells from the apical papilla. International Endodontic Journal. 49(10): 950-959. [PubMed.]
- Wang F, Jiang Y, Huang X, Liu Q, Zhang Y, Luo W, et al. (2017) Pro-inflammatory cytokine TNF-α attenuates BMP9-induced osteo/odontoblastic differentiation of the stem cells of dental apical papilla (SCAPs). Cellular Physiology and Biochemistry. 41(5): 1725-1735. [PubMed.]
- Singer NG, Caplan AI. (2011) Mesenchymal stem cells: mechanisms of inflammation. Annual Review of Pathology: Mechanisms of Disease. 6: 457-478. [PubMed.]
- Prockop DJ, Oh JY. (2012) Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Molecular therapy. 20(1): 14-20. [PubMed.]
- Karp JM, Teo GS. (2009) Mesenchymal stem cell homing: the devil is in the details. Cell stem cell. 4(3): 206-216. [PubMed.]
- Rustad KC, Gurtner GC. (2012) Mesenchymal stem cells home to sites of injury and inflammation. Advances in wound care. 1(4): 147-152. [PubMed.]
- Vishwanat L, Duong R, Takimoto K, Phillips L, Espitia CO, Diogenes A, et al. (2017) Effect of bacterial biofilm on the osteogenic differentiation of stem cells of apical papilla. Journal of endodontics. 43(6): 916-922. [PubMed.]
- Lin LM, Shimizu E, Gibbs JL, Loghin S, Ricucci D. (2014) Histologic and histobacteriologic observations of failed revascularization/revitalization therapy: a case report. Journal of endodontics. 40(2): 291-295. [PubMed.]
- Mohammadi Z, Jafarzadeh H, Shalavi S, Yaripour S, Sharifi F, Kinoshita JI. (2018) A review on triple antibiotic paste as a suitable material used in regenerative endodontics. Iranian endodontic journal. 13(1): 1-6. [Ref.]
- Gunasekaran S, Sakthivel S, Babu G, Vijayan V. (2021) Clinical Application of Platelet-Rich Fibrin in Pediatric Dentistry. Journal of Health and Allied Sciences NU. 12(02): 186-190. [Ref.]
- Chen YP, Jovani‐Sancho MD, Sheth CC. (2015) Is revascularization of immature permanent teeth an effective and reproducible technique? Dental Traumatology. 31(6): 429-436. [PubMed.]
- Torabinejad M, Nosrat A, Verma P, Udochukwu O. (2017) Regenerative endodontic treatment or mineral trioxide aggregate apical plug in teeth with necrotic pulps and open apices: a systematic review and meta-analysis. Journal of endodontics. 43(11): 1806-1820. [PubMed.]
- Tong HJ, Rajan S, Bhujel N, Kang J, Duggal M, Nazzal H. (2017) Regenerative endodontic therapy in the management of nonvital immature permanent teeth: a systematic review—outcome evaluation and meta-analysis. Journal of endodontics. Sep 1) 43(9): 1453-1464. [PubMed.]
- Chen MH, Chen KL, Chen CA, Tayebaty F, Rosenberg PA, Lin LM. (2012) Responses of immature permanent teeth with infected necrotic pulp tissue and apical periodontitis/abscess to revascularization procedures. International endodontic journal. 45(3): 294-305. [PubMed.]
- Alobaid AS, Cortes LM, Lo J, Nguyen TT, Albert J, Abu-Melha AS, et al. (2014) Radiographic and clinical outcomes of the treatment of immature permanent teeth by revascularization or apexification: a pilot retrospective cohort study. Journal of endodontics. 40(8): 1063-1070. [PubMed.]
- Kahler B, Mistry S, Moule A, Ringsmuth AK, Case P, Thomson A, Holcombe T. (2014) Revascularization outcomes: a prospective analysis of 16 consecutive cases. Journal of endodontics. 40(3): 333-338. [PubMed.]
- Lei L, Chen Y, Zhou R, Huang X, Cai Z. (2015) Histologic and immunohistochemical findings of a human immature permanent tooth with apical periodontitis after regenerative endodontic treatment. Journal of endodontics. 41(7): 1172-1179. [PubMed.]
- Shabahang S, Torabinejad M, Boyne PP, Abedi H, McMillan P. (1999) A comparative study of root-end induction using osteogenic protein-1, calcium hydroxide, and mineral trioxide aggregate in dogs. Journal of endodontics. 25(1): 1-5. [PubMed.]
- Mitchell PJ, Ford TP, Torabinejad M, McDonald F. (1999) Osteoblast biocompatibility of mineral trioxide aggregate. Biomaterials. 20(2): 167-173. [PubMed.]
- Torabinejad M, Hong CU, McDonald F, Ford TP. (1995) Physical and chemical properties of a new root-end filling material. Journal of endodontics. 21(7): 349-353. [PubMed.]
- Torabinejad M, Chivian N. (1999) Clinical applications of mineral trioxide aggregate. Journal of endodontics. 25(3): 197-205. [PubMed.]
- Maeda H, Nakano T, Tomokiyo A, Fujii S, Wada N, Monnouchi S, et al. (2010) Mineral trioxide aggregate induces bone morphogenetic protein-2 expression and calcification in human periodontal ligament cells. Journal of endodontics. 36(4): 647-652. [PubMed.]
- Tezel B, Uysal S, Turgut M, Cehreli Z. (2010) Inadvertent MTA extrusion in an immature traumatized permanent incisor. Journal of Clinical Pediatric Dentistry. 35(2): 145-148. [PubMed.]
- Guven G, Cehreli ZC, Ural A, Serdar MA, Basak F. (2007) Effect of mineral trioxide aggregate cements on transforming growth factor β1 and bone morphogenetic protein production by human fibroblasts in vitro. Journal of endodontics. 33(4): 447-450. [PubMed.]
- Sari Ş, Durutűrk L. (2007) Radiographic evaluation of periapical healing of permanent teeth with periapical lesions after extrusion of AH Plus sealer. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 104(3): e54-59. [PubMed.]
- Azim AA, Griggs JA, Huang GJ. (2016) The Tennessee study: factors affecting treatment outcome and healing time following nonsurgical root canal treatment. International endodontic journal. 49(1): 6-16. [PubMed.]
- Galler KM, Widbiller M, Camilleri J. (2021) Bioceramic Materials in Regenerative Endodontics. Bioceramic Materials in Clinical Endodontics. 29-38. [Ref.]
- Özdemir O, Kopac T. (2022) Cytotoxicity and biocompatibility of root canal sealers: A review on recent studies. Journal of Applied Biomaterials & Functional Materials. 20: 22808000221076325. [PubMed.]
- Silva EC, Tanomaru-Filho M, da Silva GF, Delfino MM, Cerri PS, Guerreiro-Tanomaru JM. (2020) Biocompatibility and bioactive potential of new calcium silicate–based endodontic sealers: Bio-C Sealer and Sealer Plus BC. Journal of Endodontics. 46(10): 1470-1477. [PubMed.]
- Bryan TE, Khechen K, Brackett MG, Messer RL, El-Awady A, Primus CM, et al. (2010) In vitro osteogenic potential of an experimental calcium silicate–based root canal sealer. Journal of endodontics. 36(7): 1163-1169. [PubMed.]
- Lee BN, Hong JU, Kim SM, Jang JH, Chang HS, Hwang YC, et al. (2019) Anti-inflammatory and osteogenic effects of calcium silicate–based root canal sealers. Journal of endodontics. 45(1): 73-78. [PubMed.]
- Karkoutly M, Alnassar I, Laflouf M, Bshara N. (2022) Management of failed regenerative endodontic treatment of a necrotic immature molar: a case report with six-month follow-up. Authorea Preprints. [Ref.]