>Corresponding Author : Mohammed Assaad Alnafie
>Article Type : Case Report
>Volume : 4 | Issue : 1
>Received Date : 22 Dec, 2023
>Accepted Date : 09 Jan, 2024
>Published Date : 12 Jan, 2024
>DOI : https://doi.org/10.54289/JCRMH2400105
>Citation : Alnafie MA, Moualek S and Ghebriout B. (2024) Bilateral High-Riding Jugular Bulb and Jugular Diverticulum Dehiscents into the Mastoid cells and Trautmann’s Triangle. J Case Rep Med Hist 4(1): doi https://doi.org/10.54289/JCRMH2400105
>Copyright : © 2024 Alnafie MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access
1Faculty of Medicine of Oran, University of Oran 1 Ahmed Ben Bella
2Department of Anatomy, University Hospital Center of Oran
*Corresponding author: Mohammed Assaad Alnafie, Faculty of Medicine of Oran, University of Oran 1 Ahmed Ben Bella
Abstract
High-riding jugular bulb is a common abnormality that involves the jugular bulb. It corresponds to a jugular bulb higher than a specific anatomical landmark, which is still controversial. A diverticulum may grow up from the jugular bulb dome in different directions. The jugular bulb may be well-corticated or, on the contrary, dehiscent when the bony cover is deficient. All these anatomical conditions may still be asymptomatic or provoke debilitating symptoms or surgical complications. In this case report, we present a 30-year-old man who suffered from debilitating vertigo and pulsatile tinnitus that hindered his quality of life. Temporal bone high-resolution computed tomography revealed bilateral high-riding jugular bulb, right jugular diverticulum, and right-side dehiscence into the mastoid cells and Trautmann’s triangle. The patient was managed conservatively but without significant symptom relief. This case report highlights an uncommon presentation of dehiscent high-riding jugular bulb and jugular diverticulum that, to the best of our knowledge, has not been reported before.
Keywords: High-riding jugular bulb, Jugular bulb diverticulum, Dehiscence, Vertigo, Pulsatile tinnitus, Hearing loss
Abbreviations: JB: Jugular Bulb, SS: Sigmoid Sinus, IJV: Internal Jugular Vein, JBD: JB Diverticulum, RW: Round Window, IAC: Internal Auditory Canal, HRCT: High-Resolution Computed Tomography, VA: Vestibular Aqueduct, PSCC: Posterior Semicircular Canal, IAC: Internal Auditory Canal, ES: Endolymphatic Sac, FN: Facial Nerve, MD: Meniere's Disease, EH: Endolymphatic Hydrops
Introduction
The jugular bulb (JB) is the dilated venous junction that connects the sigmoid sinus (SS) to the internal jugular vein (IJV) [1-3] and occupies the pars vascularis of the jugular foramen [2]. A close relationship exists between the JB and surrounding structures, such as the middle and inner ears and cranial nerves [2,4]. High-riding JB (HRJB), dehiscent HRJB (DHRJB), and JB diverticulum (JBD) are JB abnormalities [5,6]. The threshold that determines an HRJB is still controversial. This threshold may be the basal turn of the cochlea (BT), the inferior margin of the round window (RW), the inferior bony annulus, or the internal auditory canal (IAC) [5,7]. When the bony wall covering the JB is deficient, it is called dehiscent [7]. HRJB is common and often asymptomatic but sometimes provokes hearing loss, vertigo, pulsatile tinnitus, facial paralysis, or bleeding during ear surgery [3,5,8]. However, JBD is rare and may have different projection directions [5]. Several options are used to treat an HRJB, but conservative management is favored instead of surgery [7]. During a surgical procedure, the surgeon must remain vigilant when manipulating JB to avoid inadvertent injury that may lead to profuse hemorrhage [3]. The surgeon must check for this condition on clinical and radiological exams to keep the patient safe. We report the case of a young man suffering from vertigo and pulsatile tinnitus. His clinical condition was related to a bilateral HRJB and JBD associated with dehiscence into the mastoid cells and the Trautmann triangle.
Case report
A 30-year-old man consulted an oto-rhino-laryngologist for debilitating pulsatile tinnitus and rotatory vertigo that deprived him of sleep and disrupted his work. He also reported hearing loss, temporal headaches, fluctuating otalgia, and neck pain. He denied otorrhea or symptoms that suggest ear or respiratory infection. His medical history included hiatal hernia, lower limb osteoid osteoma, and allergic rhinitis. He had no history of recent trauma, addiction, ototoxic medication, visual troubles, or psychological disorders. Upon clinical exam, the Romberg test was positive, and the patient presented an unbalanced gait that worsened when the eyes were closed. Otoscopic, neurological, ophthalmic, nasal, and oral exams were unremarkable. Then, pure-tone audiometry showed a mild conductive hearing loss for 4000 Hz frequency sounds in the right ear with an air-bone gap of 20 dB (Figure 1). The tympanometry showed a type A tympanogram in both ears. So, the patient underwent a temporal bone high-resolution computed tomography (HRCT) that showed the superior margin of the right JB reached the lower border of the RW (Figure 2A, 2B). It was facing the tympanic cavity at the level of the hypotympanum, covered by an intact bony wall, and did not protrude into the middle ear (Figure 2C, 2D). In addition, a JBD extended from the right JB between the vestibular aqueduct (VA) and mastoid cells, reaching the posterior semicircular canal (PSCC) (Figure 3A-3C). The JB and the diverticulum were dehiscents into mastoid cells and Trautmann’s triangle (Figure 4, Figure 5). The left JB reached the level of the BT (Figure 6). Head-contrast CT showed normal blood flow bilaterally in the sigmoid sinuses, jugular bulbs, and jugular veins (Figure 7). Inner ear anomaly, intracranial aneurysm, vascular malformation, and persistent embryologic vessels were excluded. Cervical spine X-ray showed sagittal straightness and right-sided frontal deviation, but no cervical-occipital hing anomaly was observed in the cervical CT (Figure 8). Therefore, the patient was diagnosed with a right DHRJB and dehiscent JBD and left HRJB. After a discussion with the patient, the oto-rhino-laryngologist proposed a conservative management. During the three-year follow-up, no hearing deterioration was noticed, but there was no remission of vertigo and tinnitus.
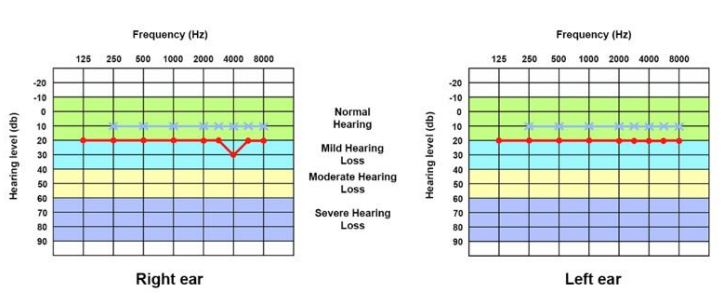
Figure 1. Pure-tone audiogram of both ears. In the right ear, there is mild conductive hearing loss for 4000 Hz frequency sounds with an air-bone gap of 20 dB.
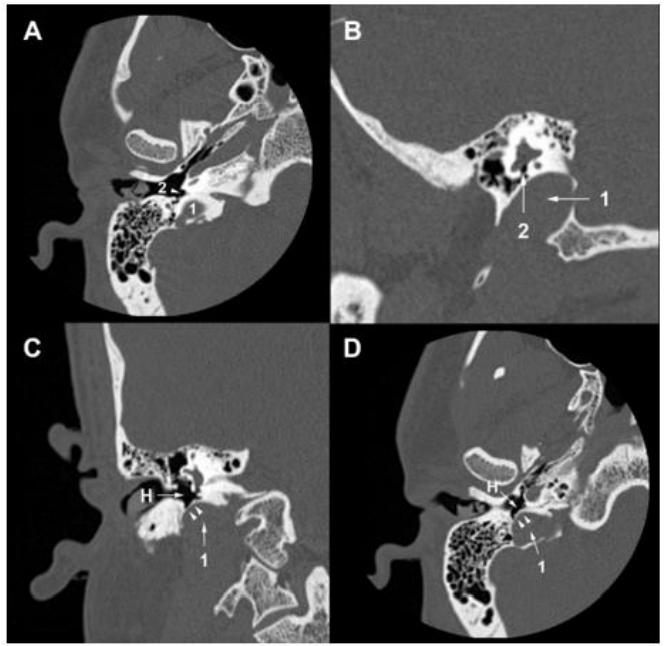
Figure 2. Temporal bone HRCT. (A) (B) The right jugular bulb (1) reaches the lower border of the round window (2). (C) (D) The right jugular bulb (1) faces the hypotympanum (H), covered by an intact bony wall (arrowheads).
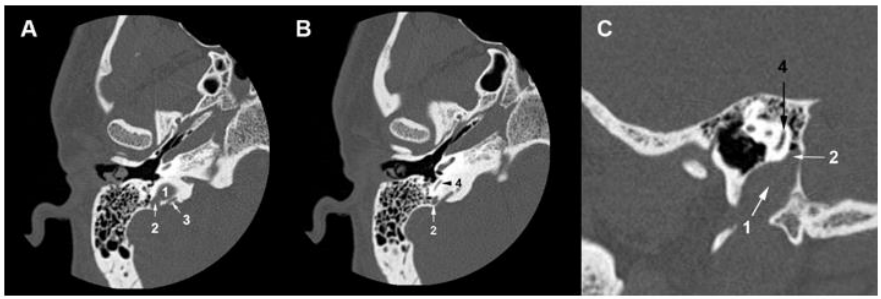
Figure 3. (A) Temporal bone HRCT (A) A jugular diverticulum (2) grows from the right jugular bulb (1) and extends between the vestibular aqueduct (3) and mastoid cells. (B) (C) Axial and sagittal temporal bone HRCT slices show that the jugular diverticulum (2) reaches the level of the posterior semicircular canal (4).
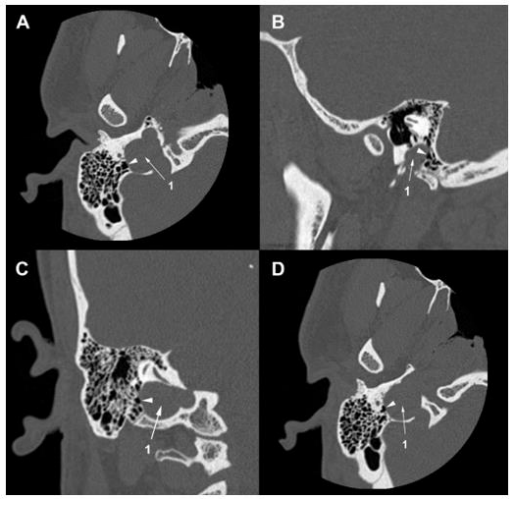
Figure 4. Temporal bone HRCT. (A) (B) (C) (D) The right jugular bulb (1) is dehiscent into mastoid cells (arrowhead).
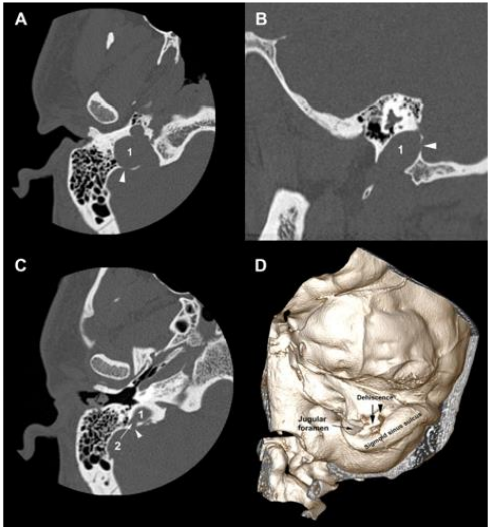
Figure 5. Temporal bone HRCT and 3D CT. (A) (B) The right jugular bulb (1) is dehiscent into Trautmann’s triangle (arrowhead). (C) The jugular diverticulum (2) is dehiscent into Trautmann’s triangle (arrowhead). (D) 3D CT of the right temporal bone shows the bony dehiscence in Trautmann’s triangle area.
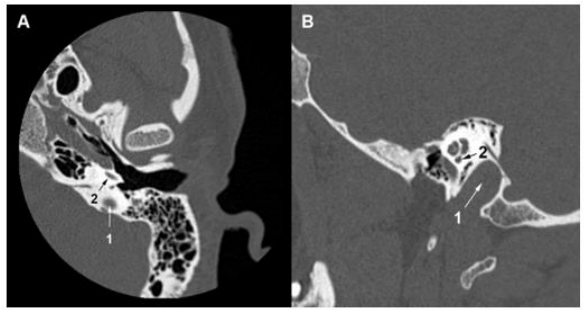
Figure 6. Temporal bone HRCT. (A) (B) The left jugular bulb (1) reaches the basal turn of the cochlea (2).
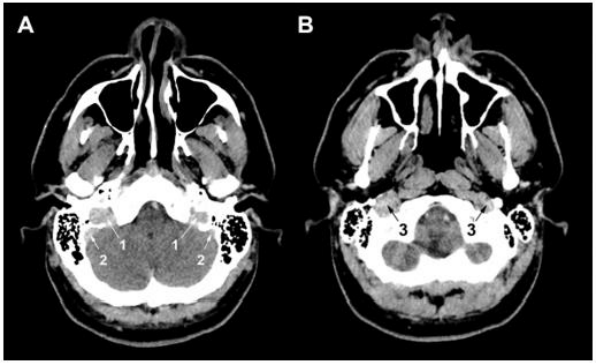
Figure 7. Head contrast CT. (A) (B) A regular blood flow is observed in jugular bulbs (1), sigmoid sinuses (2), and jugular veins (3).
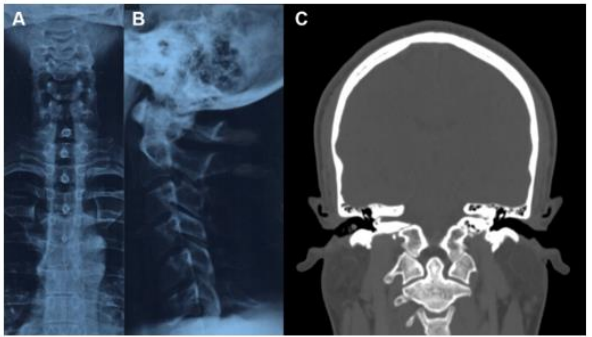
Figure 8. Cervical X-ray and head and neck CT. (A) A right-sided frontal deviation of the cervical spine is observed in the frontal view. (B) The cervical spine presents a rectitude observed in the sagittal view. (C) No anomaly of the cervical-occipital hing is observed.
Discussion
The petrous portion of the temporal bone can present several vascular variations, the most common of which is the HRJB [3,8]. The definition of the HRJB is controversial since the authors disagree about the ear structure to consider as a landmark. Some authors proposed the inferior margin of the internal auditory canal (IAC) [8], while others used the BT, the jugular foramen apex [2,3], the hypotympanum, or the tympanic annulus [2]. Thus, the JB is high-riding if it exceeds one of these elements. Sasindran et al. assert that the JB is high-riding if it invades the hypotympanum. Singla et al. suggested two diagnosis parameters to define an HRJB. The first is a distance between the JB dome and the RW or the IAC less or equal to 2mm. The second is a zero distance between the jugular fossa and the endolymphatic sac (ES) opening [2]. In addition, if the bony wall separating the JB from the surrounding structures is defective, the JB is called dehiscent [3]. Thus, a herniation may occur in the middle ear, auditory canal, cochlea, VA, facial nerve (FN) canal, or PSSC [1]. Further, an outpouching growing from the JB dome is called JBD. Some authors consider it a venous anomaly, while others consider it a JB that extends inside [2].
Okudera et al. reported that JB does not appear before age two [3,8,9]. They studied aborted fetuses and children up to six years and speculated that, during the fetal period and first two years, bony and cartilaginous structures surrounding the JB prevent its development [9]. Beyond age two, the standing posture provokes negative pulses in the cranial venous circulation, enlarging the IJV-SS junction, forming the JB, and excavating the jugular fossa [1,9]. The mechanism leading to an HRJB is still unknown. It may relate to the blood flow imbalance in the head venous system. Indeed, it may explain the right-side dominance of the HRJB. An embryologic asymmetry of the dural sinuses and a longer left brachiocephalic vein could increase the negative pulses (cited above) on the right side [8]. In addition, Kao et al. demonstrated that, parallel to an HRJB, a helical flow existed in the IJV [2]. Mastoid pneumatization affects the JB position [2,4,8] since it affects the location and curve of the SS [4]. Sufficient pneumatization places the SS deep behind, and its vertical limb continues as the JB after a gentle S-curve: the JB is low. Vis versa, poor pneumatization pushes the SS upward: the JB is high [4]. As speculated by Lyu et al., a correlation exists between the JB position and the semicircular canals’ orientation [2]. Age and gender may influence HRJB development since the frequency and size change with them. Friedmann et al. reported that the prevalence of HRJB was 1.7% in patients younger than 10, increased with puberty and between the ages of 30 and 50, beyond which it stabilized [1]. Wang et al. observed a decrease in the prevalence beyond age 60 and linked that to bony resorption attenuation and hormone level changes related to age [8]. However, this hypothesis seems not to be always true. Friedmann et al. reported cases were younger than five [1]. Aksoy et al. found, in their series, that 53% of children with HRJB were younger than two years, while 86% were older than 12 months. The earliest case they found was eight months old [9]. Such observations suggest HRJB may be either congenital or it develops early. In the Zuniga study, 46.7% of patients with HRJB were younger than two years, and there was no significant difference across age groups [3]. Wang et al. observed more HRJB in women, while Friedmann et al. and Zuniga et al. did not find a significant difference between genders [1,3,8]. Next, they remarked that the mediolateral dimension was wider in all HRJB specimens; the anteroposterior axe was wider only in dehiscent HRJB. So, they deduced that HRJB grows first in the mediolateral axe and then in the anteroposterior one. In addition, there was no significant difference, in terms of width, between HRJB and DHRJB. So, the dehiscence depends on JB dimensions and relationships, which depend on mastoid pneumatization and temporal development [1]. HRJB and JBD may erode several structures, such as the cochlea, VA, PSCC, IAC, and tympanic cavity [1-3].
The literature reports a divergence between studies on the prevalence of HRJB, JBD, and DHRJB. In their series of 2299 temporal CT, Woo et al. have considered the JB as high riding if it reaches any point between the RW and the IAC. Thus, they reported 9.5% and 2.6% respective prevalence of HRJB and DHRJB. Atmaca et al. studied the HRJB in 1010 Turkish participants who underwent temporal CT, using the IAC as a landmark. They found HRJB and DHRJB in 15.2% and 7.5% of cases [3]. Friedmann et al. evaluated 100 temporal CTs and 1579 temporal bone specimens, considering the definition of Atmaca et al. They found an 8.2% prevalence in the histological sample and an 8.5% prevalence in the radiological sample. Then, DHRJB was found in 2.8% of CTs and 3% of temporal specimens, while the JBD was found in 1.3% [1]. Zuniga et al. studied, retrospectively, bilateral temporal CT of 229 Mexican patients between two months and 91 years old. They used the basal turn of the cochlea as a landmark to define the HRJB. The prevalences of HRJB and DHRJB were 38.4% and 1.3%, more often in women. Further, across-age frequency matched that of the Friedmann study. Wang et al. reported that HRJB existed in 14.5% of 4539 healthy Chinese participants who underwent a head MRI, of which 1.5% had a bilateral HRJB [8]. Aksoy et al. reported an equivalent frequency of bilateral HRJB in 194 children [9]. According to Chen et al. and Huang et al., DHRJB occurred in 0.5–1.7% [7]. To summarize, we agree with Wang et al. [8] that as long as the HRJB definition lacks consistency, its prevalence will still be imprecise. Otherwise, the sample size, the study design, the research techniques, and other factors could influence the results [8].
HRJB can be asymptomatic or provoke vertigo, headaches, ear fullness, conductive or sensorineural hearing loss, and pulsatile tinnitus [2,3,8]. Conductive hearing loss involves HRJB protrusion against the eardrum and obstruction of the RW. HRJB may block sound transmission through the tympano-ossicular chain by impeding its mobility. In addition, a JB protruding in the inner ear through the RW or compressing the vestibulocochlear nerve may generate sensorineural hearing loss and tinnitus [8]. The literature proposes that HRJB is a risk factor for Meniere's disease (MD) since it provokes MD-like symptoms [3]. The association between HRJB-related VA dehiscence and endolymphatic hydrops (EH), the substratum of MD, is reported [2]. Some authors speculated that HRJB prevents endolymphatic resorbing [9] and subsequently leads to EH. However, Haginamori et al. reported, in their series of 197 temporal bones, that 2% of HRJB specimens had VA dehiscence without EH. Histologically, they found a hypoplastic ES running through a hypoplastic VA resulting probably from JB-related ES compression. In previous work, Friedmann et al. studied 30 patients with DJB confirmed in HRCT, of which 25 had VA dehiscence, and two-thirds were symptomatic; only two patients had EH. They studied simultaneously 1579 temporal bone specimens, of which 41 had VA dehiscence, and reported just two cases of associated EH [1]. Globally, the studies conflict about the association between EH and HRJB; Therefore, it is early to admit the role of HRJB in MD. The HRJB appears in the otoscopic exam as a bluish mass behind the posterior inferior quadrant of the eardrum. All actions increasing the JB pressure, such as the Valsalva maneuver, ipsilateral IJV compression, and supine position, will distend it and facilitate the exam [2,4,7]. When HRJB manifests as a bluish mass, the differential diagnosis involves vascular conditions that appear the same, such as internal carotid artery aberrance or diverticulum, eardrum phlebectasia, and hemangioma. Other tumors may be similar: blue-domed mucosal cyst, paraganglioma, aural polyp, and extramedullary hematopoiesis [7]. HRJB may be confused with other vascular conditions such as glomus tympanicum or cholesterol granuloma. A well-corticated HRJB may look like a cholesteatoma or neoplasm [10]. However, a negative otoscopic exam does not exclude the diagnosis since the JB may fluctuate in size from one examination to another [4]. In addition, an opacified eardrum, as in chronic middle ear disease, may hide the mass [7]. Likewise, the JBD does not, classically, protrude in the tympanic cavity and, consequently, is still invisible. Thus, its diagnosis is often incidental. The HRCT is the gold standard for diagnosing and evaluating the HRJB and JBD. This latter appears as an expansion of the JB extending, commonly, upward and inside the petrosal bone [2]. When the HRJB is asymptomatic, an inadvertent surgical injury may lead to a profuse hemorrhage [7]. Thus, HRCT or angiography evaluation is necessary prior to surgery [3]. Manjila and Semaan proposed a classification of the HRJB based on the HRCT (Table 1). It allows the surgeons to accurately foresee the potential risk of bleeding during surgery [2].
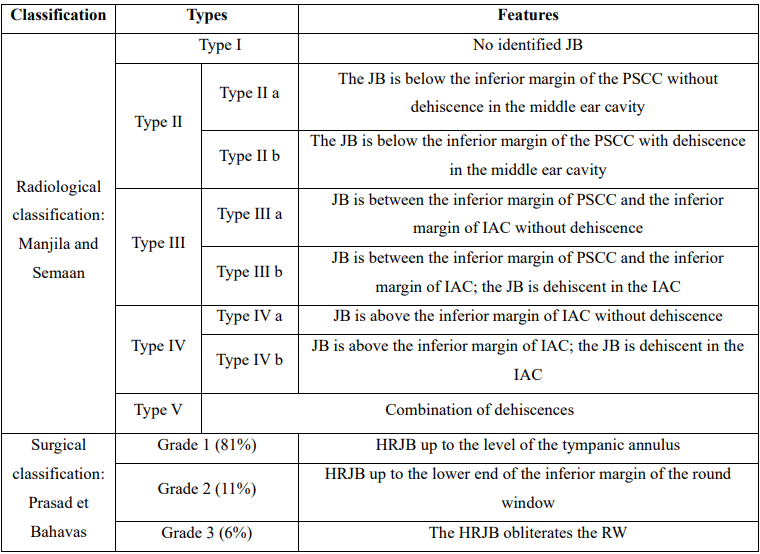
Table 1. Classification of the HRJB according to Manjila et al. [5] and Prasad et al. [6]
Although it does not prohibit ear surgery [2], HRJB is a potential danger because of eventual hemorrhagic and air embolism complications [3,8]. These events may occur if the JB is below the tympanic annulus, during the removal of osteomyelitis bone, keratin from the EAC, or tumors. It also occurs during the transcanal decompressing of the FN [4]. In addition, the surgeon must be careful during stapedectomy or tympanoplasty when elevating the tympanomeatal flap [4]. Effectively, he must follow the fibrous annulus and never attempt a biopsy [10]. If the HRJB is above, the injury may occur during the translabyrinthine approach or IAC surgery. During mastoid or labyrinthine surgery, a backward HRJB may disturb the intervention [4]. Manjila and Seeman's classification offers a good compromise between safety and efficiency. It allows surgeons to prepare surgical procedures and estimate their results. The exposure of SS and JB is necessary for skeletonization. When HRJB is of type I and II of Manjila, access to the posterior fossa is easier through the translabyrinthine approach. However, when it is of a type III or IV of Manjila, the access is blocked, and the JB may be injured because of its high position. The retrosigmoid approach is better for reaching the cerebellopontine angle but is risky during IAC exposure [2]. If a patient had this type of HRJB and MD, we must verify the vacuity of the VA. If obliterated, the VA separates the ES from the rest of the labyrinthine fluid space, and the shunting/decompression of the ES will fail [4]. Prasad et al. proposed another classification of the HRJB based on surgical observation of 49 HRJB (Table 1). In grades III to V, the HRJB hampers the RW reflex test. The surgeon should push the JB and remove the covering epithelium with utmost care [10]. In addition, a retro-labyrinthine HRJB blocks access to the IAC and limits the exposure of the cochlear aqueduct [8,10]. In such a situation, a translabyrinthine approach is impossible [10]. Several treatment options are proposed for a symptomatic HRJB. First, we can use medical procedures such as labyrinthine sedation and control of systemic hypertension [2]. Surgical management is considered for an HRJB that provokes severe symptoms, such as annoying tinnitus, intensive vertigo, or progressive hearing loss. As proposed by Buckwalter et al., we can ligate the IJV when tinnitus is debilitating [2,7]. Despite its successful outcomes, such a procedure exposes neck vital vascular and neural structures to significant risk [7]. In addition, this ligation may disrupt the venous flow and lead to idiopathic intracranial hypertension [2]. Next, the literature reports endovascular access as an alternative to surgery. The therapist either deploys a stent in the JB or coils it [2,7]. Trivelato et al. reported good outcomes after stent deployment and selective embolization of a JBD in a patient suffering from pulsatile tinnitus. Mortimer et al. used a transfemoral approach to treat two JBDs, the first by deploying stent and coil and the second with coiling only. The patient had a total resolution of symptoms after 10 months of follow-up. However, thrombosis occurred in the first JBD after five months. In addition, this method has a high rate of intracranial hypertension and ischemic stroke. For a DHRJB, literature proposed several surgical techniques to replace the JB and reconstruct the covering bone. In the first technique, the surgeon performs a mastoidectomy and applies a bone wax to cover the JB; the FN, middle ear, and dura mater are at risk of injury during such a procedure [2]. Glasscock et al. treated two cases of JBD protruding in the tympanic cavity, causing hearing loss. They reinstated the JB and then used a piece of mastoid bone as a graft to cover it. Despite a successful procedure, the patients did not recover their auditive function. Therefore, Glasscock et al. did not recommend this technique. El-Begermy et al. used bone dust, perichondrium, and tragal cartilage in multiple layers to rebuild the bony dehiscence on a series of seven patients complaining of tinnitus. Five had symptom relief, and one had intracranial hypertension [7].
In our case, we encountered a unique anatomical presentation of the JB during the diagnosis and assessment of our patient’s disorders. First, we objectified a bilateral HRJB associated with a right JBD; as reported above, they are rare entities. Then, the literature includes only one case of JBD-mastoid dehiscence in a 30-year-old man, reported by Fukumoto et al. In our case, both HRJB and JBD were dehiscent in the mastoid cavity. Next, the new is the dehiscence of both right JB and JBD in Trautmann’s triangle. To the best of our knowledge, there is no similar case reported before. Trautmann’s triangle is a surgical triangle between the JB, sinodural angle, and PSCC. This triangle allows access to the cerebellopontine angle and cranial nerves V, VII, VIII, IX, and X during the retro-labyrinthine presigmoid approach. This approach served for vestibular neurectomy to treat vertigo and to resect lesions ventral to the brainstem, such as petroclival meningioma. We use it currently to shunt the ES in MD [8]. The dehiscence in Trautmann’s triangle with imminent herniation of the JB may disturb such surgical procedures.
Conclusion
The HRJB is an underestimated abnormality since the definition’s consistency is lacking. It provokes annoying symptoms such as vertigo, tinnitus, and hearing loss; however, it is still often asymptomatic and bleeds during ear surgery. Therefore, surgeons must check for this abnormality and its anatomical variant to preserve the patient’s safety; the HRCT represents the best option. Then, they must be vigilant during the surgery for fear of injuring the JB inadvertently. In conclusion, we believe that the case we report deserves to be shared since it illustrates a new demonstration of DHRJB and DJBD not reported before.
Consent for publication: The patient has given his consent for the publication of this clinical case.
Ethical approval: This case report has been performed in accordance with the ethical standards as laid down in the Declaration of Helsinki and the ethical standards of the ethical and deontological committee of the University of Oran 1 Ahmed Ben Bella
Availability of data and material: All data generated or analyzed during this study are included in this published article.
Competing interests: Authors have declared that no competing interests exist
Funding: No funding has been received by the author for preparing this work
References
- Friedmann DR, Eubig J, Winata LS, Pramanik BK, Merchant SN, et al. (2012) Prevalence of jugular bulb abnormalities and resultant inner ear dehiscence: A histopathologic and radiologic study. Otolaryngol Head Neck Surg. 147: 750-756. [PubMed.]
- Manjila S, Bazil T, Kay M, Udayasankar UK, Semaan M. (2018) Jugular bulb and skull base pathologies: proposal for a novel classification system for jugular bulb positions and microsurgical implications. Neurosurg Focus. 45: 1-8. [PubMed.]
- Irabien-Zuñiga M, Gonzalez-Treviño M, Pinales-Razo R, et al. (2022) Prevalence of high riding jugular bulb and dehiscence: an evaluation using computed tomography. Med Univ. 24: 19-24. [Ref.]
- Graham MD. (1977) The jugular bulb: its anatomic and clinical considerations in contemporary otology. Laryngoscope. 87: 105-125. [Ref.]
- Fukumoto I, Yamasaki K, Yonekura S, Iinuma T, Mita Y, et al. (2021) A case of jugular bulb diverticulum causing pulsatile tinnitus. Clin Case Rep. 9: 1-4. [Ref.]
- Tubbs RS, Griessenauer C, Loukas M, Ansari SF, Fritsch MH, et al. (2014) Trautmann’s triangle anatomy with application to posterior transpetrosal and other related skull base procedures. Clin Anat. 27: 994-998. [PubMed.]
- Shaikh MF, Mahboubi H, German M, Djalilian HR. (2013) A novel approach for surgical repair of dehiscent high jugular bulb. Laryngoscope. 123: 1803-1805. [PubMed.]
- Wang J, Feng Y, Wang H, et al. (2020) Prevalence of high jugular bulb across different stages of adulthood in a chinese population. Aging Dis. 11: 770-776. [PubMed.]
- Aksoy SH, Yurdaisik I. (2023) High riding jugular bulb: prevalence and significance in asymptomatic children. Acta Radiol. 64: 792-797. [PubMed.]
- Prasad KC, Basava CH, Gopinathan PN, Induvarsha G, Harshita RT, et al. (2018) A revisit to high jugular bulb: a newer clinical grading. Indian J Otolaryngol Head Neck Surg. 70: 527-530. [PubMed.]