>Corresponding Author : Isabella Pia Palmieri
>Article Type : Original Research Article
>Volume : 4 | Issue : 4
>Received Date : 03 Feb, 2024
>Accepted Date : 14 Feb, 2024
>Published Date : 19 Feb, 2024
>DOI : https://doi.org/10.54289/JCRMH2400116
>Citation : Palmieri IP and De Luca C. (2024) Striae Distensae and an Innovative Intradermal Medical Device Based on PN HPT™, Hyaluronic Acid, and Mannitol. A Real-World Insight. J Case Rep Med Hist 4(4): doi https://doi.org/10.54289/JCRMH2400116
>Copyright : © 2024 Palmieri IP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Original Research Article | Open Access
1Public Health Department, Federico II University of Naples Medical School, Naples, Italy
2Carmen De Luca Beauty Clinic, Cassino, Italy
*Corresponding author: Isabella Pia Palmieri, Public Health Department, Federico II University of Naples Medical School, Naples, Italy
Abstract
Introduction: Striae distensae are often more than a mere skin aesthetic trouble for their impact on self-esteem and as signals of internal medicine disorders like hypercortisolism or cachexia. There is a need to advance in treating striae distensae effectively, particularly their late atrophic hypochromic stage. PN HPT™ may help to restore and preserve a physiologically sound dermal environment. The paper reports the outcomes of a survey study based on an anonymous questionnaire. The study’s purpose was to confirm the long-term effectiveness and safety profile of an intradermal medical device based on PN HPT™ (1 mg/mL), HA (1 mg/mL), and mannitol ) within a multi-year clinical monitoring program.
Methods: The investigators — dermatologists and plastic and aesthetic surgeons — carried out the study in real-world subjects, all of them women, who spontaneously sought office treatment to improve skin quality in areas of subjectively grievous striae distensae and striae albae—a total of 38 lesions. All investigators had already used the investigated intradermal device for short-term treatment and long-term maintenance. All surveyed subjects had undergone a cycle of six to eight intradermal injection sessions over three months to improve the texture of their striae distensae—a once-weekly injection for the first month and one injection every two weeks for the last two months with a tolerance of two missing injections. Assessment tools and timing: Global Improvement Scale (GIS) and Global Aesthetic Improvement Scale (GAIS) two to four weeks after the end of the treatment period.
Results: At the follow-up visit, the investigators evaluated that, globally (mean GIS scores), 50.0% and 47.4% of the treated striae distensae showed moderate improvement or were much improved compared with the baseline. The corresponding outcomes for the independent evaluator were 55.3% and 44.7%. The local skin quality and texture in the lesion areas (mean GAIS scores) appeared to have improved or to be much improved by 50.0% and 50.0% according to investigators, and by 42.9% and 57.1%, respectively, according to surveyed patients, with no reported side effects.
Conclusion: The real-world monitoring survey demonstrated that the NEWST ONE medical device based on PN HPT™, HA, and mannitol as an inhibitor of HA metabolism retains its long-standing record of efficient performance and excellent safety with no variations over time.
Keywords: PN HPT™; Polynucleotides; Hyaluronic Acid; Mannitol; Real-World Study; Skin Quality
Abbreviations: GIS: Global Improvement Scale, GAIS: Global Aesthetic Improvement Scale, CDLQI: Children’s Dermatology Life Quality Index, PN HPT: Polynucleotides Highly Purified Technology, HA: Hyaluronic Acid
Introduction
Improving the aesthetics of the impervious forms of dermal scarring known as striae distensae has been a medical challenge since the first histological description in 1889 [1]. The treatment challenge does not ease following the progressive fading of the earlier, inflamed erythematous and violaceous striae rubrae into the finely wrinkled and atrophic dermal scar-like lesions known as striae albae. Unfortunately, the striae albae problem, prevalent in epidemiological studies between 11% and 88%, is extensive in the general population. It is not simply a problem of adolescents fearful of peers’ judgment or young pregnant women unsatisfied with their self-image or physical appearance [2].
The words of a recent paper about the adverse impact of striae distensae, either erythematous or atrophic, on adolescents 9-12 and 13-16 years old leave no doubt—“ (striae distensae) should not be considered only as a cosmetic problem” [3]. The psychosocial impact is severe, as measured by the Children’s Dermatology Life Quality Index (CDLQI) and does not differ in the two adolescent age groups investigated in the study, with median total scores of only 7 out of a maximum score of 30 in younger adolescents and 6 in the 13 to 16 age group [3].
The emotional and psychological impact on pregnant women is much like that on adolescents, with the midwifery care effort to prevent striae gravidarum through moisturizing measures correlating with the emotional impact scores [4,5].
Recent research has cast light on striae distensae as diagnostic signals and possibly even pathogenetic precursors of psychiatric conditions—typically, eating disorders. Anorexia nervosa, bulimia nervosa, binge eating, and related anxiety and depression disorders would act as a form of compensatory behavior subterfuge after discovering the dermal scarring lesions [6]. Striae distensae, as markers of improper connective tissue deposition and scaffolding, are also diagnostic signals of patients with connective tissue disorders like Marfan’s syndrome or internal medicine conditions like hypercortisolism or cachexia [2].
In summary, striae distensae are more than a mere skin aesthetic trouble, with a clear need to advance in their management and treatment and, even more, of their late atrophic hypochromic stage.
Previous evidence suggests that Polynucleotides Highly Purified Technology (PN HPT™) may be an innovative option for managing striae distensae and the striae albae late stage [7-10]. PN HPT™ rapidly fill the intradermal hollow spaces. At the same time, in the background, PN HPT™, which also have free radical scavenger properties, help preserve a physiologically sound dermal environment [10-12]. PN HPT™ act in the scarred dermis through spontaneous degradation and passive replenishment of the fibroblast pool of nitrogen bases and nucleotide precursors [11,13,14]. The long-term outcome is to support fibroblast viability in the skin areas with dystrophic and atrophic striae distensae.
The PN HPT™ impact on the scarred dermal environment is more potent than hyaluronic acid (HA) and synergizes with HA in combined formulations [11,13,14]. The PN HPT™ benefits on dermal viability and physiology have long been exploited in aesthetic medicine and chronic wound and ulcer management [12-17].
The paper reports the outcomes of the survey study based on an anonymous questionnaire. The investigators — dermatologists and plastic and aesthetic surgeons — carried out the survey study in real-world subjects, all women, who spontaneously sought office treatment to improve skin quality in areas of subjectively grievous striae distensae and striae albae. The subjects were prospectively enrolled with the guarantee to remain anonymous and minimal inclusion and exclusion criteria to simulate a real-world situation as far as possible.
All investigators had already used the investigated PN HPT™/HA/mannitol intradermal device according to regulatory prescriptions, including the suggested long-term maintenance treatment—one PN HPT™/HA/mannitol syringe every one or two months [9]. The survey study was the first within a long-term program to monitor the persistent efficacy performance, safety, and lack of emergent risks of a Class III CE-mark medical device, already available on the market, for intradermal injections with PN HPT™, HA, and mannitol as key ingredients. Confirming the profile of known side effects and contraindications and identifying unknown or emergent side effects and risks was another purpose of the study and the long-term monitoring program. Resorting to real-world data without the rigid inclusion and exclusion criteria to select patients in randomized clinical studies supports the study value.
Materials and Methods
Survey study design — Conceived as a single-arm cohort of adults of both genders prospectively enrolled in a real-world setting. The candidate subjects should have voluntarily sought specialist help to relieve their subjectively troublesome burden of striae distensae and improve skin quality. Before the survey, all enrolled subjects had undergone a cycle of six to eight intradermal injection sessions over three months—once-weekly injection for the first month and one injection every two weeks for the last two months (complete protocol with a tolerance of two missing injections).
The Class-III CE-marked medical device used in the pre-survey treatment sessions (NEWEST ONE, Mastelli S.r.l., Sanremo, Italy) contains a sterile isotonic solution of polynucleotides PN HPT™ (1 mg/mL) and HA (1 mg/mL) in 4-mL apyrogenic prefilled disposable syringes with 30G½ needles for intradermal injection. A third ingredient, mannitol, is an inhibitor of HA degradation. NEWEST ONE is indicated to moisturize the tissue, improve the turgidity, elasticity, and appearance of the skin, and promote the remodeling of areas with a strong fibrous component and depressed scarring, such as striae distensae, as part of treatment protocols customized by the doctor.
The survey study after the voluntary therapy sessions was purely observational, with no other active intervention and assessments at baseline and end of treatment for efficacy and throughout the study period for safety. The questionnaire-based format allowed investigators to answer questions thoroughly and faithfully without time constraints and more quickly than face-to-face interviews.
Efficacy assessments
Primary efficacy endpoint: Comparison by the investigators and an independent evaluator of the skin-quality improvements compared with baseline in the monitored striae distensae areas, based on their expert evaluation of photographs documenting the baseline situation before the first therapy session and the skin changes two to four weeks after the end of the treatment cycle. Semi-quantitative assessment: based on the five-score quartile Global Improvement Scale (GIS, Table 1), developed in 2010 to evaluate the effect of laser treatment of striae distensae and used in several clinical studies on striae distansae [18,19].
Secondary efficacy endpoint: Overall improvement in skin appearance two to four weeks after the last NEWEST ONE therapy session compared to pre-treatment, subjectively assessed by the investigator and self-assessed by the surveyed subject, using the five-score quartile Global Aesthetic Improvement Scale (GAIS, Table 2) [20].
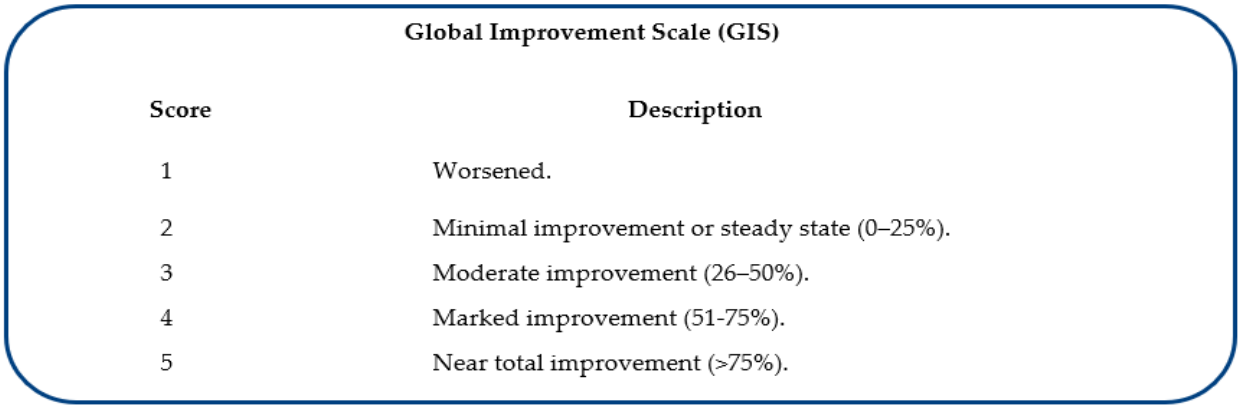
Table 1: Rating scores and descriptors of the GIS assessment instrument (evaluation of the global skin appearance) by the investigators and an independent evaluator [18,19].
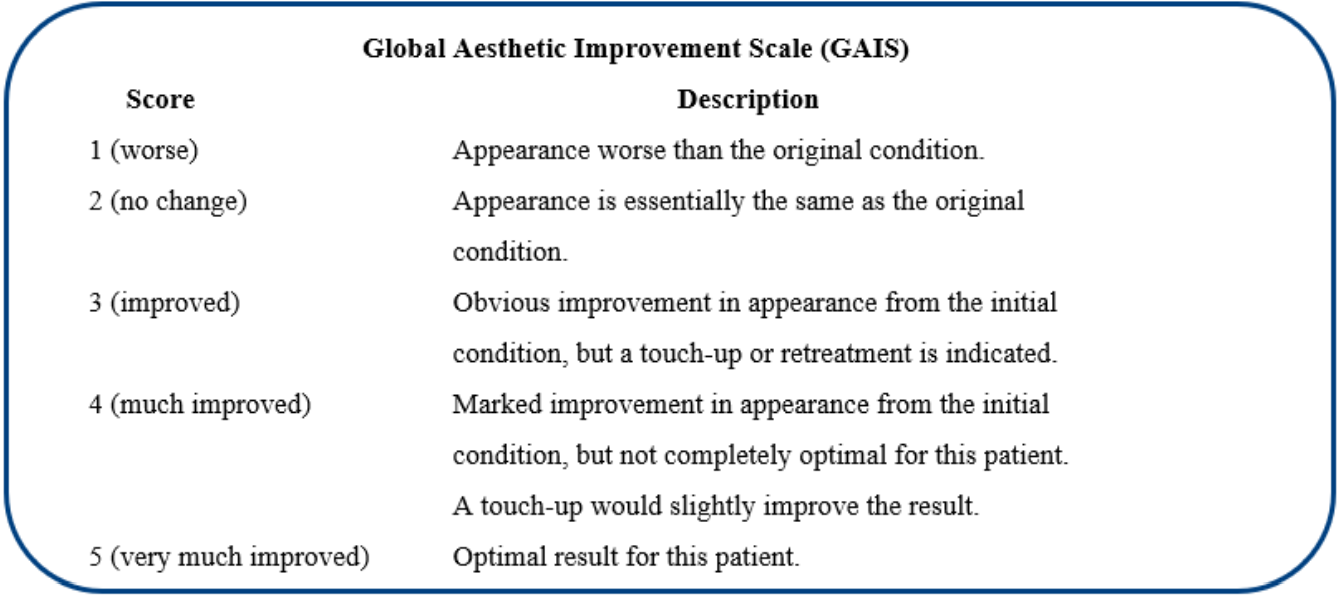
Table 2: Rating scores and descriptors of the GAIS instrument (evaluation of the local skin quality and texture in the lesion areas) [20].
Beyond being validated, the five-score GIS and GAIS assessment instruments offer a further statistical benefit. Outcomes evaluated on limited-score scales have unimodal and symmetric distributions; conversely, scales with a higher number of scores have highly skewed J and U-shaped distributions. Outcomes assessed on limited-score scales also have lower means and floor and ceiling effects. At the same time, regression analysis shows that assessment scales with few scores account for a significant fraction of total variance in floor and ceiling effects and minimize the contribution of unaccounted factors [21].
Observational safety assessments
Based on spontaneous reporting by cohort individuals and open questions in the questionnaire, the purpose was to identify known side effects and describe their presentation and severity using an impromptu three-score qualitative scale (“mild”, “moderate”, and “severe”), and identify any previously unknown adverse event or emergent risk. The investigator complemented the individual spontaneous reports by actively questioning for adverse events at the final assessment visit. Reporting: as percent of subjects.
Statistics
The sample size was estimated using the G*Power statistical program version 3.14. The sample size calculation assumed a conservative 40% improvement in the mean investigator’s GIS score after the treatment cycle. Under this assumption, the statistical power to detect a two-tailed significant improvement and minimize the ß-risk of false-negative type II errors would have been greater than 0.92 with a sample of 34 striae distensae [22].Inferential statistics was limited to compare the mean investigator’s and independent evaluator’s GIS score distribution at the end of the follow-up period (2x2 χ2 test for proportions with 5% significance level; statistical program: StatPlus release v7) [23].
Results
Table 3 illustrates the surveyed subjects’ demographics and the topographic distribution of the 38 treated striae.
Figure 1 shows the cohort’s distribution of infiltration techniques. According to the clinicians who performed the study (Figure 2), 97% of the striae improved more than 25% compared with baseline, while 47% of the atrophic lesions had an improvement higher than 50%. Only a few subjects (2.6%) had a minimal improvement; no surveyed subject experienced a clinical worsening of his striae distensae.
According to the independent evaluator (Figure 3), the overall outcome was similar but slightly more conservative for the highest degree of improvement (χ2 test for the outcome distribution between investigators and the independent evaluator: p <0.05). However, the improvement was judged superior to 25% for 100% of the evaluated striae distensae, with no striae distensae classified as “minimally improved”.
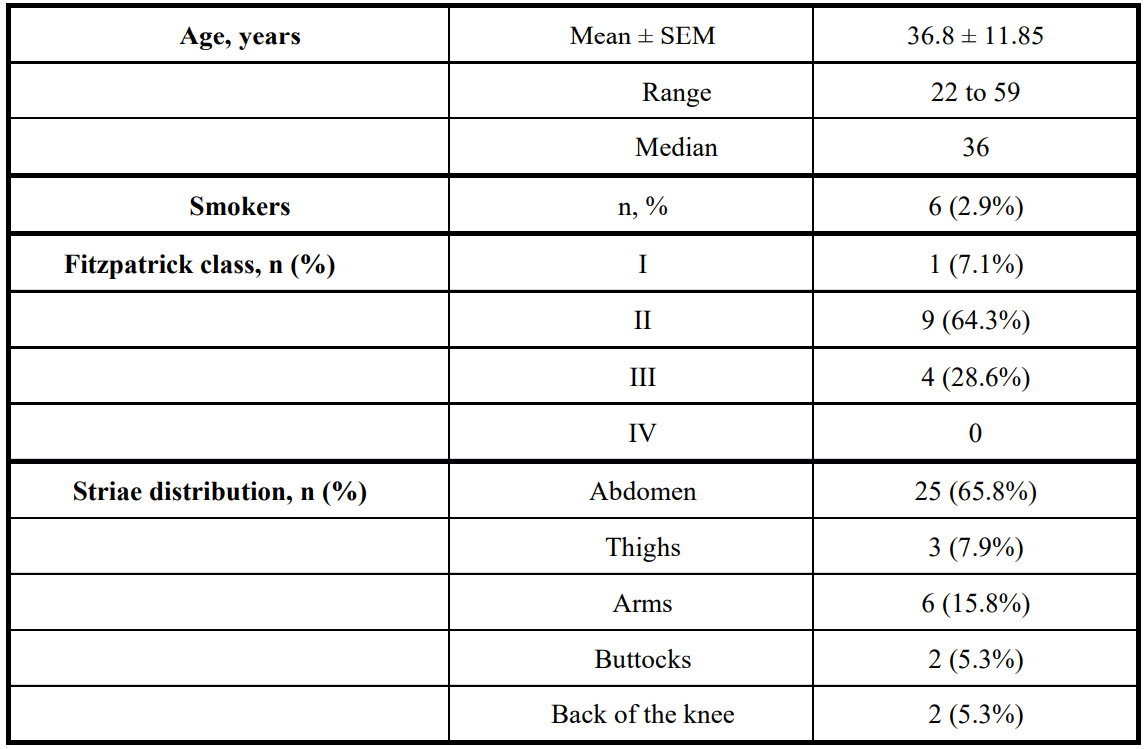
Table 3: Demographics of the subjects surveyed after treatment with NEWEST ONE. SEM = standard error of the mean
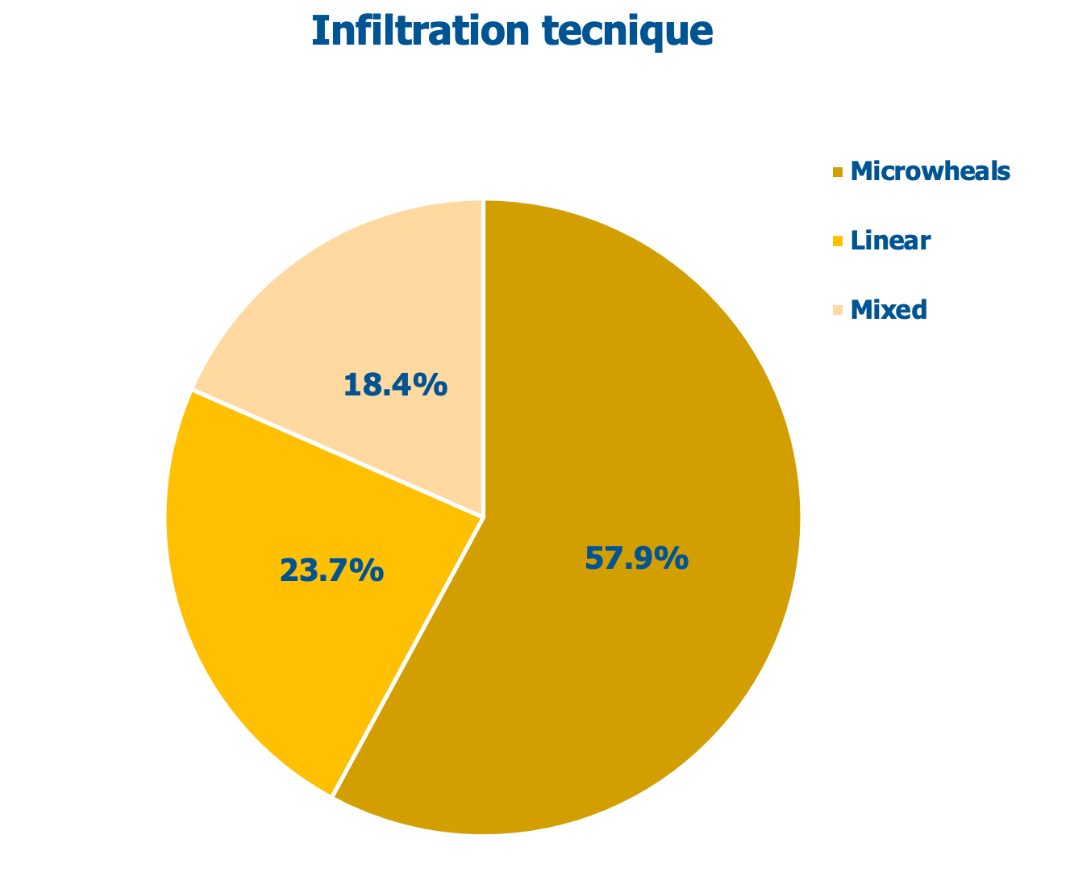
Figure 1: Techniques used for intradermal injections in surveyed subjects.
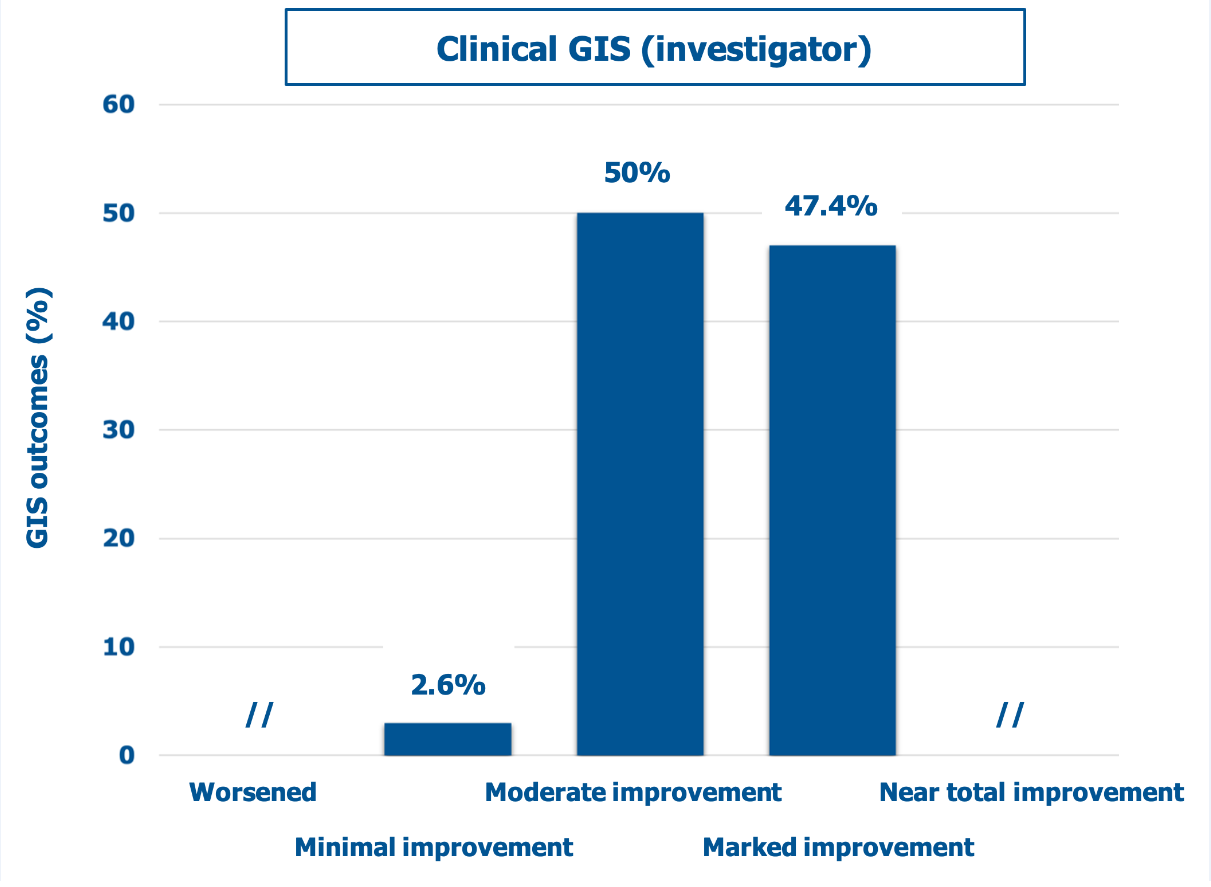
Figure 2: Percent distribution of mean GIS scores (descriptors on the abscissa) according to investigators.
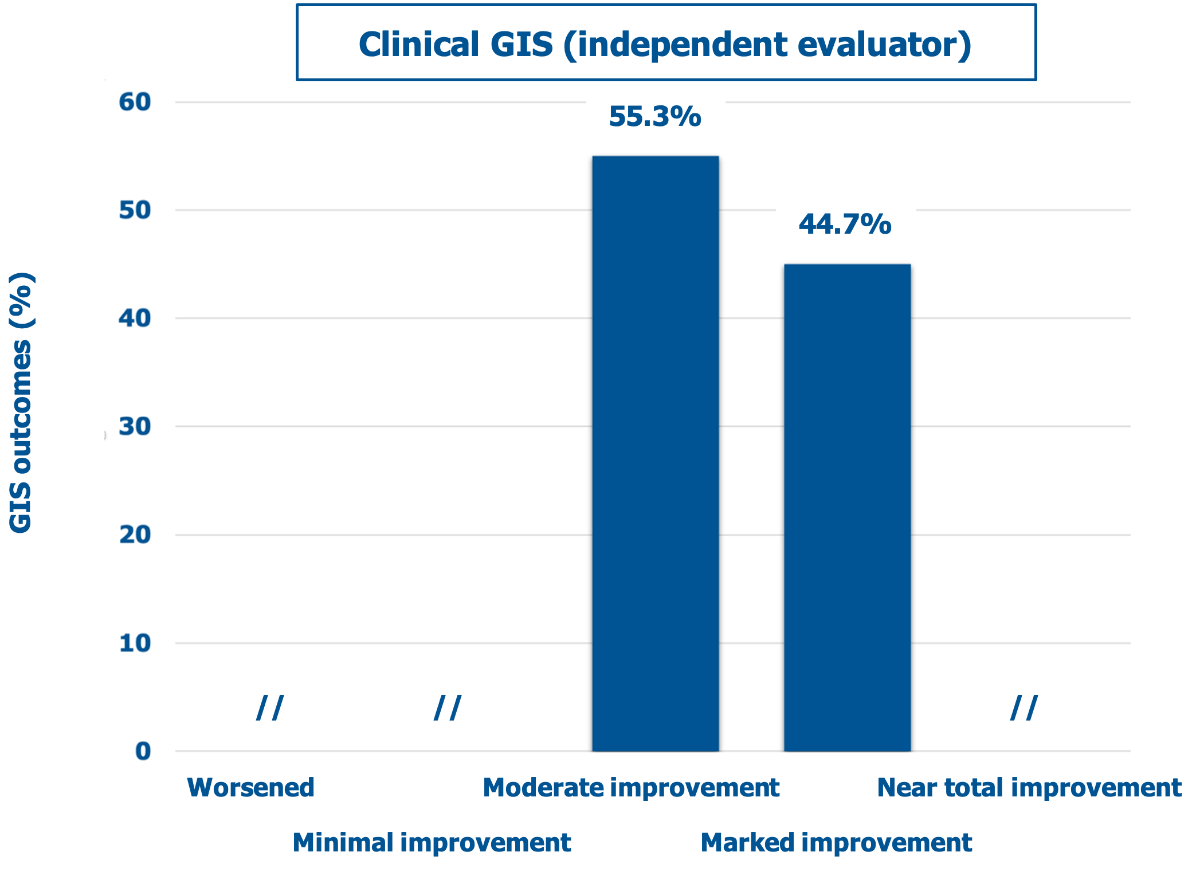
Figure 3: Percent distribution of mean GIS scores (descriptors on the abscissa) according to the independent evaluator.
Figure 4 and Figure 5 show the GAIS scores for the clinicians who performed the treatment and the treated subjects, respectively. There was no objective or subjective worsening in the dermal scarring areas. Conversely, all lesions improved or much improved objectively and subjectively at the final follow-up visit, with the treated subjects especially satisfied (57.1% rating the outcome as much improved). Figure 6 illustrates three representative examples of the skin quality benefits foreseeable after a NEWEST ONE treatment of mature striae distensae located on the abdomen (A and B) and the arm (C).
Another NEWEST ONE cycle of therapy was suggested to one subject to consolidate and further improve the benefits she had obtained.
The NEWEST ONE treatment was well tolerated, with no previously unknown adverse events and mild and transient minor side effects only at the injection site with no need for treatment—erythema in four subjects and bruising and pain at the injection site in two subjects, respectively. No subjects reported ulceration, suppuration, keloid formation, recurrence, or other severe side effects. All side effects resolved at most in a few hours; only an episode of bruising lasted for five days.
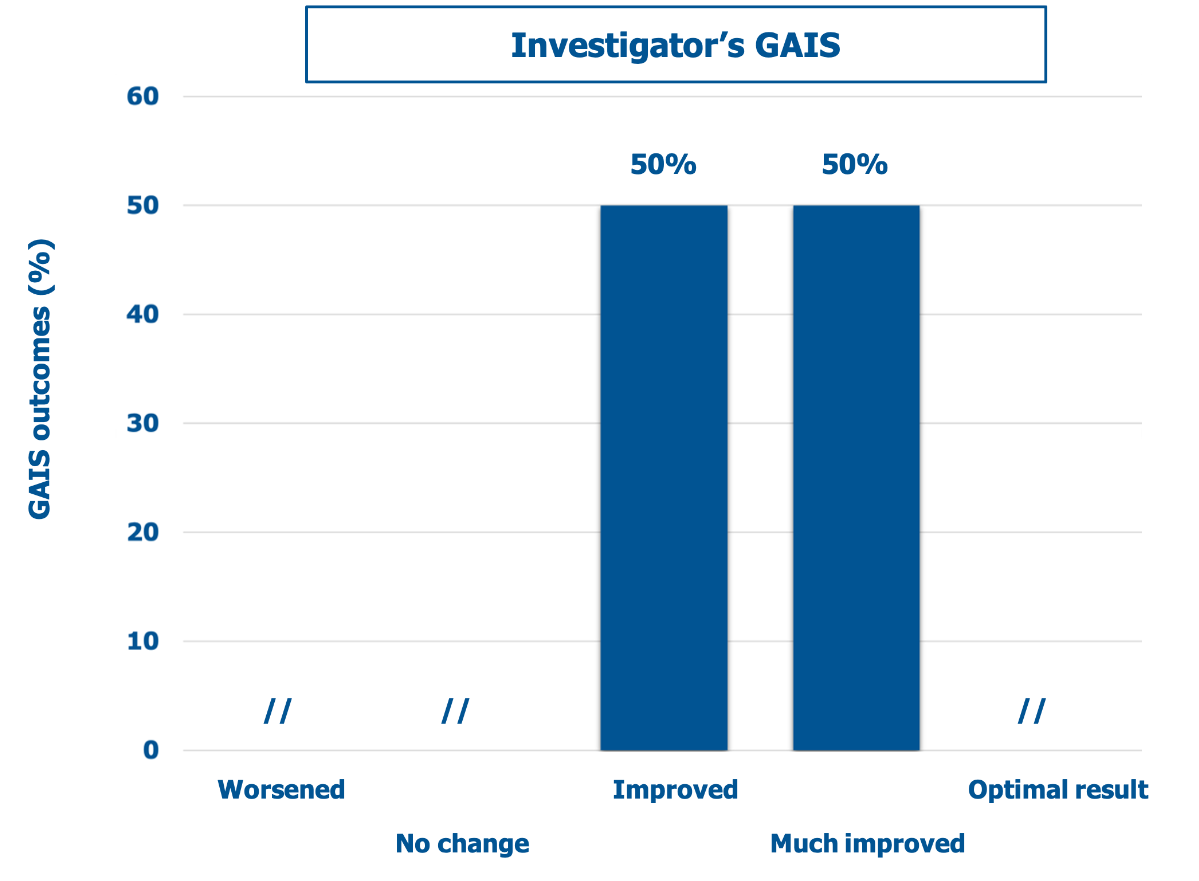
Figure 4: Percent distribution of mean GAIS scores (descriptors on the abscissa) according to the clinicians who performed the treatment (investigators).
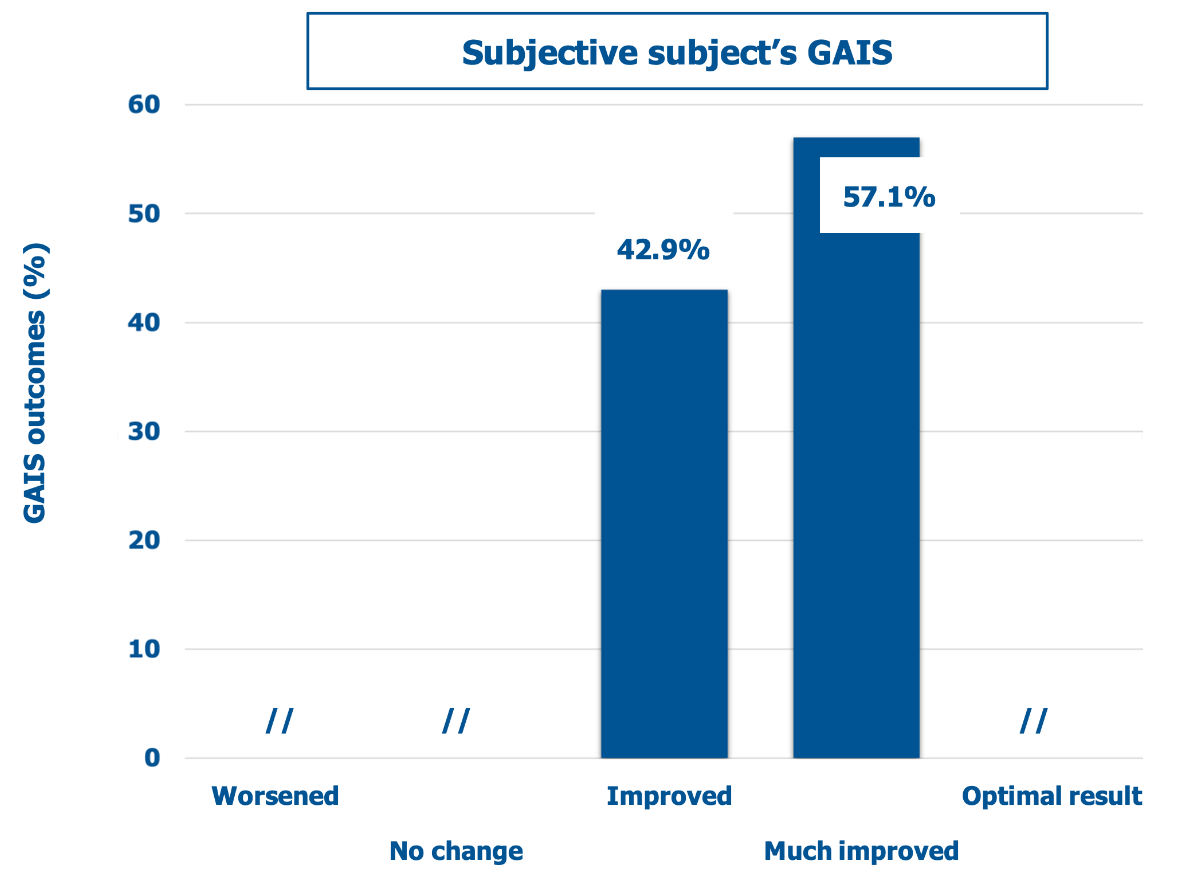
Figure 5: Percent distribution of mean GAIS scores (descriptors on the abscissa) according to the self-assessment by treated subjects.
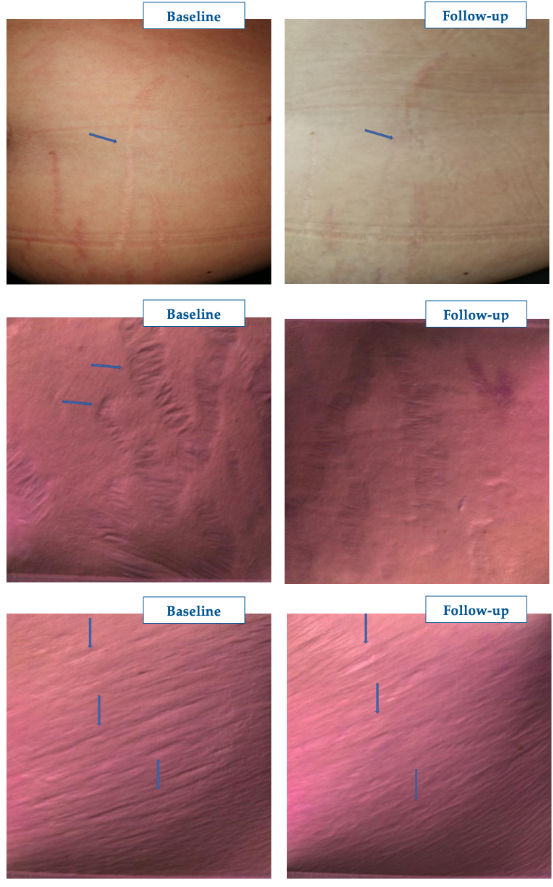
Figure 6: Representative examples of the aesthetic benefits foreseeable with NEWEST ONE. Pictures published with the subject’s written agreement. Courtesy of Isabella Pia Palmieri.
Discussion
The increasing attention to subjective well-being has led to an increased search for effective treatments for striae distensae, especially mature striae albae [24,25]. The nature, especially of the later forms, often perceived as an actual disease due to their psychosocial impact, reinforces the case for effective therapies. Still, a therapeutic strategy that fully fulfills expectations has yet to emerge. There are abundant studies with conflicting outcomes in the medical literature, with topical treatments like tretinoin and energy-based devices radiofrequency, intense pulsed light, or infrared phototherapy, Er:YAG (Erbium-doped Yttrium Aluminum Garnet), diode, Q‑switched Nd:YAG (pulsed Neodymium-doped Yttrium Aluminum Garnet), pulse dye, and excimer lasers [24-26]. Similarly inconclusive are the studies with platelet-rich plasma and chemical peels, aluminum oxide microdermabrasion, micro-needling, carboxytherapy, and galvanopuncture. Outcomes are conflicting even with the ablative fractional 10,600-nm CO2 laser, the current treatment gold standard for striae albae [24-26].
PN HPT™ reorganize three-dimensionally in the dermal scarring areas, with a primary purpose analogous to the cited unreliable treatment strategies—acting as potent yet passive fibroblast activators, facilitating the deposition of new collagen and proelastin fibers in a newly produced dermal matrix [24,25]. PN HPT™ lack all toxicological liabilities at the clinically administered doses [27]. HA, the second NEWEST ONE ingredient, synergically increases volumes under the depressed striae distensae [10-12].
The study outcomes, albeit short-term as appropriate for its monitoring purpose, are based on the validated GIS and GAIS instruments. With no significant side effects other than a few mild episodes of mild irritation and pain at the injection site, the study outcomes support the concept that drove the study design—the NEWEST ONE combination of PN HPT™, HA, and mannitol led to a clinically meaningful correction of the striae in all subjects and looks like an efficient treatment strategy for striae distensae including troublesome mature striae albae.
Acknowledgements
Mastelli S.r.l, Sanremo, Italy, produces the injectable PN HPT™/hyaluronic acid/mannitol gel formulation tested in this real-world study within a long-time program to monitor the device’s persisting efficacy and safety. The authors acknowledge the contribution of Mastelli S.r.l. for supporting the publication costs.
References
- Troisier E, Ménétrier P. (1889) Histologie des vergetures. Ann Gynecol. 31: 206. [Ref.]
- Al-Himdani S, Ud-Din S, Gilmore S, Bayat A. (2014) Striae distensae: a comprehensive review and evidence-based evaluation of prophylaxis and treatment. Br J Dermatol. 170(3): 527-547. [PubMed.]
- Aşkın Ö, Özçakır EC, Uzunçakmak TK, Kutlubay Z, Serdaroğlu S. (2021) Evaluation of quality of life in children and adolescents diagnosed with striae distensae. Turk Arch Pediatr. 56(5): 447-450. [Ref.]
- Yamaguchi K, Suganuma N, Ohashi K. (2014) Prevention of striae gravidarum and quality of life among pregnant Japanese women. Midwifery. 30(6): 595-599. [PubMed.]
- Karhade K, Lawlor M, Chubb H, et al. (2021) Negative perceptions and emotional impact of striae gravidarum among pregnant women. Int J Womens Dermatol. 7(5Part B): 685-691. [PubMed.]
- Silva V, Schukow CP, Restini CBA. (2022) Striae distensae as a diagnostic indicator for eating disorder pathologies. Int J Dermatol. 62(6):715-722. [PubMed.]
- Cavallini M, Papagni M. (2007) Long-chain polynucleotides gel and skin biorevitalization. J Plastic Dermatol. 3(3): 27-32. [Ref.]
- Matera G, Dodici G, Raichi M. (2020) Improving on laser: biorevitalization of stretch marks, the polynucleotides infiltrations combined with CO2 laser option. Aesth Med. 6(2): 7-24. [Ref.]
- Bartoletti E, Trocchi G. (2020) Polynucleotides Highly Purified Technology, the new class of body skin biorevitalizing agents. Aesth Med. 7(1): 41-45. [Ref.]
- Cavallini M, Bartoletti E. (2021) on behalf of The Polynucleotides HPT™ Priming Board, Board, Collegio Italiano delle Società Scientifiche di Medicina Estetica (Italian College of the Aesthetic Medicine Scientific Societies) — SIME, AGORÀ, SIES. Consensus report on the use of PN HPT™ (Polynucleotides Highly Purified Technology) in aesthetic medicine. J Cosmet Dermatol. 20(3): 922-928. [PubMed.]
- Bartoletti E, Cavallini M, Maioli L, et al. (2020) Introduction to Polynucleotides Highly Purified Technology. Aesth Med. 6(2): 43-47. [Ref.]
- Colangelo MT, Govoni P, et al. (2021) Polynucleotide biogel enhances tissue repair, matrix deposition, and organization. J Biol Regul Homeost Agents. 35(1): 355-362. [PubMed.]
- Cavallini M, De Luca C, Prussia G, Raichi M. (2023) PN HPT™ (Polynucleotides Highly Purified Technology) in facial middle third rejuvenation. Exploring the potential. Mastelli Srl Internal R&D Report, Sanremo (Italy). 21(2):615-624. [PubMed.]
- Palmieri IP, Raichi M. (2022) Clinical commentary about foreign body complications over 20 years after polymethyl-methacrylate face implants and control of late sequelae with Polynucleotides Highly Purified Technology (PN HPT™). J Cosmet Dermatol. 21(11): 5537-5542. [PubMed.]
- Araco A, Araco F. (2021) Preliminary prospective and randomized study of highly purified polynucleotide vs. placebo in treatment of moderate to severe acne scars. Aesthet Surg J. 41(7): NP866-NP874. [PubMed.]
- De Caridi G, Massara M, Acri I, et al. (2014) Trophic effects of polynucleotides and hyaluronic acid in the healing of venous ulcers of the lower limbs: a clinical study. Int Wound J. 13(5): 754-758. [PubMed.]
- Segreto F, Carotti S, Marangi GF, et al. (2020) The use of acellular porcine dermis, hyaluronic acid, and polynucleotides in the treatment of cutaneous ulcers: single-blind randomized clinical trial. Int Wound J. 7(6): 1702-1708. [Ref.]
- Park KY, Kim HK, Kim SE, et al. (2012) Treatment of striae distensae using needling therapy: a pilot study. Dermatol Surg. 38(11): 1823-1828. [PubMed.]
- Tabaie SM, Nasr E, Naderi MS, et al. (2018) Treatment of striae distensae using fractional ablative CO2 laser in skin types II-IV: a retrospective case series study. J Cosmetic Laser Ther. 20(6): 330-334. [PubMed.]
- Lemperle G, Holmes RE, Cohen SR, Lemperle SM. (2001) A classification of facial wrinkles. Plast Reconstr Surg. 8(6): 1735-1750. [PubMed.]
- Garratt AM, Helgeland J, Gulbrandsen P. (2011) Five-point scales outperform 10-point scales in a randomized comparison of item scaling for the Patient Experiences Questionnaire. J Clin Epidemiol. 64(2): 200-207. [PubMed.]
- Faul F, Erdfelder E, Buchner A, et al. (2009) Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 41(4): 1149-1160. [Ref.]
- (2023) Statistical software. [Ref.]
- Mimi R Borrelli, Michelle Griffin, Ledibabari Mildred Ngaage, et al. (2021) Striae distensae: scars without wounds. Plast Reconstr Surg. 148(1): 77-87. [PubMed.]
- Adam Hague, Ardeshir Bayat. (2017) Therapeutic targets in the management of striae distensae—a systematic review. J Am Acad Dermatol. 77(3): 559-568.e18. [Ref.]
- Nicholas A Ross, Derek Ho, Juliya Fisher, et al. (2017) Striae distensae: preventative and therapeutic modalities to improve aesthetic appearance. Dermatol Surg. 43(5): 635-648. [PubMed.]
- (2023) Injectable gel medical devices toxicological assessment, KROS BioScience Healthcare consultants, Pomezia, Italy. [Ref.]