>Corresponding Author : Graham Wilson
>Article Type : Mini Review
>Volume : 4 | Issue : 7
>Received Date : 05 May, 2024
>Accepted Date : 16 May, 2024
>Published Date : 20 May, 2024
>DOI : https://doi.org/10.54289/JCRMH2400131
>Citation : Chiong HS and Wilson G. (2024) The Ocular Chloramphenicol Story. J Case Rep Med Hist 4(7): doi https://doi.org/10.54289/JCRMH2400131
>Copyright : © 2024 Chiong HS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Mini Review | Open Access
1Consultant Ophthalmologist, Dunedin Hospital, New Zealand
2Honorary Clinical Associate Professor, Gisborne Hospital, Gisborne, New Zealand
*Corresponding author: Graham Wilson, Honorary Clinical Associate Professor, Gisborne Hospital, Gisborne, New Zealand
Abstract
Chloramphenicol eye drops and ointment are the most used ocular antibiotic worldwide. Chloramphenicol was discovered in 1947 and was only the third antibiotic to successfully be produced and only the first antibiotic suitable for large-scale commercial production. By the 1950s, the drug had been developed into drop and ointment forms. The broad spectrum of anti-bacterial activity of Chloramphenicol led to widespread use. Ocular Chloramphenicol remains an important weapon in the ophthalmic armamentarium despite emergence of evidence of possible side effects in the 1970s. This is a short review of the remarkable story of the discovery, development and tribulations of ocular Chloramphenicol.
Keywords: History; Chloramphenicol
Abbreviations: FDA: Food and Drug Administration
Introduction
The research and development of the antibiotic Chloram-phenicol is of considerable relevance and interest to ophthalmology and optometry. It is only the third antibiotic in the world (after penicillin and streptomycin) to be successfully marketed by the pharmaceutical industry. It was the very first antibiotic that could be used orally or systemically [1]. When discovered in 1947, its chemical composition was considered unique because of the presence of organic bonding not previously found in naturally occurring compounds. It became the world's first antibiotic where chemical synthesis was economical and technically feasible for large-scale production. The World Health Organisation includes it on its List of Essential Medicines [2], the most important medications needed in a basic health system. The formula C11H12Cl2N2O5 (Figure 1) is the empirical formula for chloromycetin, more commonly known to us as Chloramphenicol [3].
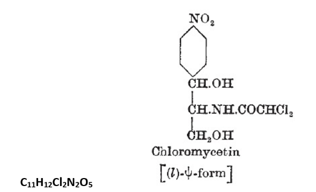
Figure 1. Empirical formula of chloromycetin
Chloramphenicol gained widespread use as a topical therapy after it became available in the 1950s because of two important properties - broad-spectrum activity and ability to penetrate the cornea [4]. Despite concerns held in North America about its safety, Chloramphenicol is the most used topical antimicrobial for eye infection around the rest of the world. For nearly seven decades Chloramphenicol has been widely available, affordable and effective. During a recent (2015) Australasian-wide supply-shortage of Chloramphenicol ointment due to manufacturing capacity, the demand for this antibiotic was overwhelming. This shortage highlighted its clinical need and the lack of a suitable alternative. Topical Chloramphenicol is an essential tool in ocular therapeutic practice. This review outlines the remarkable story behind our reliance on Chloramphenicol.
The penicillin breakthrough
The discovery of penicillin by Scottish scientist and Nobel Laureate Alexander Fleming in 1928 began the modern era of antibiotic discovery and therapeutic intervention. In 1930, Cecil George Paine, a pathologist at the Royal Infirmary in Sheffield, United Kingdom, achieved the first recorded cure with penicillin on an infant with gonococcal ophthalmia neonatorum. It was another decade before a team under Howard Florey (1945 Nobel Prize in Medicine) devised a method of mass-producing penicillin. Large scale doses of penicillin only became available in 1944, resulting in a significant reduction in the number of deaths and amputations caused by infected wounds among Allied troops fighting in Europe.
The Era of Actinomycetes
The discovery of penicillin was undoubtedly one of the greatest advances in medicine. However it was soon apparent that penicillin was ineffective against tuberculosis, the great scourge of mankind. The search for other antibiotics led scientists around the world to look for useful compounds from various microbes from bacteria and fungi to actinomycetes and plants. Rene Dubos, a French-born American microbiologist started feeding gram-positive bacteria at intervals to a large sample of soils, hoping to find microbes in the soil capable of destroying the bacteria. In 1939 he discovered a bacterium that produced an alcoholsoluble compound capable of inhibiting the growth of gram-positive bacteria. He named this newly found compound tyrothricin. It was actually a mixture of two compounds, tyrocidin and gramicidin [1]. Today these antibiotics have limited use. Nevertheless, Dubo's work inspired others and set a clear direction in the quest for novel antibiotics.
One of those inspired was Selman Waksman, a Russian-born, Jewish-American inventor, biochemist and microbiologist. His laboratory investigated microbes found in soil as a source of antimicrobials [1] and it soon became clear that the gram-positive bacteria actinomycetes were the most promising. In 1940, systematic screening of soil actinomycetes led to the discovery of actinomycin and strepthoricin [5]. These compounds proved to be too toxic for clinical use. In 1943, Albert Schatz, a graduate of Waksman's laboratory, discovered, using a similar approach, streptomycin [6].
Streptomycin was first used experimentally to treat life-threatening infections for United States Army soldiers near the end of World War II. The first patient treated did not survive; the second patient became blind as a side-effect of the treatment; and in March 1946, the third patient (Robert J. Dole, later United States Presidential nominee) experienced a rapid and robust recovery [7]. More importantly, it was found to be the first antibiotic demonstrating activity against Mycobacterium tuberculosis. The first randomized trial of streptomycin against pulmonary tuberculosis was carried out in 1946–1947 by the MRC Tuberculosis Research Unit [8] and it is widely accepted to have been the first randomized curative trial [9].
Waksman's work in general and success on different species of actinomycetes in particular proved to be sensational in the medical world. He was awarded the Nobel Prize in Physiology in 1952 and called "one of the greatest benefactors to mankind". Controversially, Schatz was overlooked for the Nobel Prize.
The discovery of Chloramphenicol
Encouraged by the commercial success of penicillin and streptomycin and to find another new antibiotic, the pharmaceutical industry initiated several major screening programs using a similar approach to that of Schatz and Waksman. Employees were sent abroad to collect soil samples. Each company had its own way of processing these soils and, as many identical soil experiments were being undertaken by a number of companies, a partnership of four American companies (Parke Davis, Eli Lilly, Abbott Laboratories and Upjohn) took place. The soil isolates were first fermented in liquid media and the resultant broths were then tested for activity against the common pathogens of human diseases. The number of tests involved was large - an average company would typically process in excess of 100,000 actinomycetes samples in a year [1].
In 1943, Parke, Davis and Company set up a collaboration with Paul Burkholder, a botanist from Yale University. Out of over 7,000 soil samples that were screened, one from a field near Caracas, Venezuela, yielded a species of actinomycetes that could produce a broad-spectrum orally active antibiotic [3]. From this, Dr David Gottlieb and colleagues at the University of Illinois isolated the active ingredient which they then named chloromycetin, in 1947. They also named the newly discovered species of actinomycetes, Streptomyces venezuelae. Parke, Davis and Company later registered the name and patented its biochemical synthesis – as a result of that registration, Chloromycetin is considered a trade name, while Chloramphenicol became its generic name.
Chloramphenicol represented a unique case in chemistry for another important reason relating to its manufacture. In general chemists find that chlorination and nitration of compounds produced agents capable of “killing organisms” such as wood preservatives or insecticides (see Figure 1). Until the discovery of Chloramphenicol, there was firm scientific belief that organisms couldn't do what chemists could (i.e. chlorination and nitration), but Gottlieb’s actinomycete could in fact do both! [10].
Over the course of his career, Gottlieb was an eminent professor of plant pathology who was a pioneer in the field of fungal physiology and antibiotics for plants. He published more than 200 research articles, wrote books, sat on editorial boards and international committees and supervised many thesis students. His students nicknamed him David "T.C." Gottlieb, the "T.C." standing for "triplicate control", famous for his approach to problem solving.
The discovery of Chloramphenicol was a clinical and commercial success that Gottlieb could never have imagined. There was a tragic twist to his life. In 1960, he was involved in a car accident which claimed the lives of his wife and two teenage children. He was the sole survivor.
The rise of Chloramphenicol
After Chloramphenicol was discovered, it was tested on known clinically relevant pathogens with exciting results. Because it could be taken orally, there were hopes that it could be the next pharmacological weapon against tuberculosis. Streptomycin had proved a disappointment since it could only be administered intramuscularly and resistance was already developing. Unfortunately, Chloramphenicol had only a weak tuberculostatic effect [11].
Chloramphenicol was widely marketed as the first broad-spectrum antibiotic that could be used both orally and topically with minimal harmful effects. By 1950, the prescribing rate of Chloramphenicol was at its peak – there were at least four large clinical trials showing positive results for its systemic use (administered orally and intravenously) against rickettsia, the pathogen responsible for typhus [3,12-14]. In one trial, twenty five proven cases of scrub typhus were successfully treated with Chloramphenicol with no reported deaths or complications [14]. The introduction of Chloramphenicol was thankfully just in time to combat scrub typhus, a classic killer of soldiers, in the Korean War.
In 1948, Parke, Davis and Company were able to identify the synthetic chemical formula of Chloramphenicol [15]. This led to its large-scale production obviating the need for fermentation. Hence, Chloramphenicol also became the world's first antibiotic where chemical synthesis was economical and technically feasible for large-scale production. The commercial success of Chloramphenicol was the biggest catalyst for Parke, Davis and Company to become one of the world's largest pharmaceutical companies.
By the mid-1950s, Chloramphenicol became available in various forms including oral tablets, oral liquid suspension, intravenous solution, ointment, and liquid eye drops. It has gained widespread use as a topical therapy for ocular infection because of its broad-spectrum activity and apparent safety profile.
Chloramphenicol and blood dyscrasia
This new broad-spectrum antibiotic was clearly showing great promise in many areas of medicine. However in 1950 a significant and alarming adverse effect attributed to Chloramphenicol emerged in the literature. Rich et al reported a case suggesting that the use of systemic Chloramphenicol had led to the development of aplastic anaemia in a patient who subsequently died [16]. Consequently in 1952, the American Food and Drug Administration (FDA) surveyed 539 case records from 37 states involving systemic Chloramphenicol [15]. They concluded that on rare occasions and in certain susceptible individuals, systemic Chloramphenicol was associated with blood dyscrasia including aplastic anaemia. Following this report, the National Research Council in America recommended that the following statement be required on the labels of all formulations for Chloramphenicol for systemic use: "Blood dyscrasia may be associated with intermittent or prolonged use. It is essential that adequate blood studies be made" [15].
After a few more reported cases of possible systemic Chloramphenicol use and blood dyscrasia, the Californian State Senate in 1969 estimated that the risk of developing aplastic anaemia was one in 36,118, which was a 13-fold higher risk than occurs naturally [17]. The study concluded that the use of systemic Chloramphenicol for prophylaxis and treatment of minor infections was unwarranted. Following this news, the clinical use and sales of systemic Chloramphenicol plummeted.
Despite “falling from favour” as a systemic treatment option, Chloramphenicol remained the most commonly prescribed topical antibiotic for ophthalmic surgical prophylaxis and superficial eye infections in the USA. The first concerns about topical ophthalmic Chloramphenicol emerged in 1965 when Rosenthal and Blackman described a reversible case of bone marrow hypoplasia which they associated with the use of Chloramphenicol eye drops [18]. The article did not have much impact on its use as an eye drop because the patient had been using it inappropriately in an unsupervised manner for 690 days. Also, the patient had a family history of a niece who had died following systemic Chloramphenicol treatment [18]. It was not until 1980, when the first fatality and then 1982, when the second fatality were reported that the safety concerns of topical Chloramphenicol use were raised again [19,20].
Within two years of Fraunfelder’s famous report of death associated with Chloramphenicol eye drops, its sales in the United States alone declined by 90% [21]. The Physicians’ Desk Reference placed a black box warning that stated "...ocular Chloramphenicol should not be used unless there is no alternative" [21]. Doctors in the United State were now in the situation of having to resort to other broad spectrum topical antibiotics. By 2013, a literature review reported a total of 23 reported cases [22] (including 12 deaths) of blood dyscrasia possibly related to the use of topical Chloramphenicol up to 1993, and 45 new case reports since [23]. However, of the 68 reported cases, less than half were actually published and some not fully investigated.
These case reports encouraged other experts outside the United States to examine the connection between this potentially fatal condition and the use of topical Chloramphenicol. Besamusca and Bastiensen in 1986, reported a retrospective study of the use of ophthalmic Chloramphenicol in the southern region of The Netherlands, with a study population of 26 500 [24]. They failed to demonstrate any link between its use and bone marrow depression. In 1998, Wilholm et al conducted a large population-based case control study, with data collected from the international granulocytosis and aplastic anaemia study involving two separate populations of 40 million people in total [25]. Among 400 cases of aplastic anaemia there was no use of Chloramphenicol eye drops. They concluded that their data provided no support to the claim that topical ophthalmic Chloramphenicol caused aplastic anaemia. Several prominent authors investigating this subject, including Fraunfelder, Isenberg and McGhee, came to very similar conclusions that the association between anaplastic anaemia and topical Chloramphenicol is a theoretical risk yet to be proven, and the estimated risk is far less than the 1 in 100,000 frequency of penicillin-induced anaphylaxis [23,26,27]. In fact Fraunfelder, whose 1982 paper had raised the alarm, later somewhat recanted his view in an article in 2013 entitled "Restricting topical ocular Chloramphenicol eye drop use in the United States. Did we over react?" His answer was "yes".
Current status of Chloramphenicol
Topical Chloramphenicol remains the most widely available, low cost, topical ophthalmic antibiotic in the world. However its weak association with aplastic anaemia still limits its use in North America where the fluoroquinolones are the preferred topical ophthalmic agents despite the attendant risk of increasing bacterial resistance. It was estimated that topical Chloramphenicol is prescribed in 55% of 'red eyes' by both ophthalmologists and general practitioners in New Zealand without any significant increase in the occurrence of blood dyscrasia) [28]. In New Zealand, there were approximately 300,000 units (ointment or drops) of Chloramphenicol sold in 2014 compared with 52,000 units of Fucithalmic (Fusidic Acid 1%) [28]. To indicate the view of New Zealand and Australian authorities that Chloramphenicol posed a minimal risk, in 2010, it was reclassified as a ‘pharmacist-only’ medicine allowing it to be sold by pharmacists over the counter without a prescription [28,29]. This resulted in an abrupt rise in sales of Chloramphenicol ointment in Australia in the following twelve months, as had also occurred in the United Kingdom [30]. The British National Formulary continues to support the use of Chloramphenicol, stating that “Chloramphenicol has a broad spectrum of action and continues to be the drug of choice for superficial eye infections” [31]. Incredibly, topical Chloramphenicol shows very little resistance, and there is speculation that this results from limited systemic usage. It is interesting that systemic Chloramphenicol is now making a comeback in usage, with the British National Formulary recommending it in infection caused by invasive Haemophilus influenzae and rickettsial infections, where tetracyclines are contra-indicated. Its systemic use should be reserved for potentially life-threatening infections, particularly those listed above [31].
The story of the ocular use of Chloramphenicol is remarkable, particularly when one considers the difference in attitude to the drug between the United States and the rest of the world. Nearly seven decades after its discovery, its properties of effectivity, low cost, wide availability, good tolerance and low resistance are of great benefit to huge numbers of patients. The concerns about Chloramphenicol-induced blood dyscrasia from topical use have largely evaporated. For ophthalmologists, optometrists and general medical practitioners, it remains the first-choice agent for superficial eye infections world-wide.
Literature Search: In 2018, the key words were searched in PubMed, google scholar and Embase.
References
- Dougherty TJ, Pucci MJ. (2012) Antibiotic discovery and development. Springer Science and Business media. New York. [Ref.]
- World Health Organisation (WHO). (2016) Essential medicines and health products, Adults 19th Edition. [Ref.]
- Raistrick H. (1949) Chloromycetin: its structure and synthesis. Nature. 553. [Ref.]
- Blogg JR. Chloramphenicol. (1991) Responsible topical use in ophthalmology. Aust Vet J. 68(1): 8-9. [PubMed.]
- Shaw PD, Ford RE. (1983) David Gottlieb, 1911-1982. Phythophathology. American Phytopathology Society. [Ref.]
- Ehrlich J, Gottlied D, Burkholder PR, Anserson LE, Prodham TG. (1948) Streptomyces Venezuelae, N. SP. The source of chloromycetin. J Bacteriol. 56(4): 467-477. [PubMed.]
- Cramer, Ben R. (1992) What it takes. New York. 110-111. [Ref.]
- Metcalfe NH. (2011) Sir Geoffrey Marshall (1887-1982): Respiratory physician, catalyst for anaesthesia development, doctor to both Prime Minister and King, and World War 1 Barge Commander. J Med Biog. 19(1): 10-14. [PubMed.]
- D’Arcy Hart P. (1999) A change in scientific approach: from alternation to randomised allocation in clinical trials in the 1940s. BMJ. 319(7209): 572-573. [Ref.]
- Horsfall JG. (1975) Paul Rufus Burkholder: 1903-1972. Biogr Mem Natl Acad Sci. Washington DC. [Ref.]
- Steenken W JR, Wulinsky E. (1950) Effects of Antimicrobial Agents on the Tubercle Bacillus and on Experimental Tuberculosis. Am J Med. 9(5): 633-653. [PubMed.]
- Green R, Mankikar DS. (1949) In vitro effects of chloromycetin on Malayan bacteria. Trans R Soc Trop Med Hyg. 43(1): 57-65. [PubMed.]
- Payne EH, Knaudt JA, Palacios S. (1948) Treatment of epidemic typhus with chloromycetin. J Trop Med Hyg. 51(4): 68-71. [PubMed.]
- Smadel JE, Woodward TE, Herbert LL, Cornelius BP, et al. (1948) Chloromycetin in the treatment of Scrub Typhus. Science. 108: 160-161. [PubMed.]
- Woodward TE, Wisseman CL. (1958) Chloromycetin: chloramphenicol. Antibiotic Monographs. Medical Encylopedia Inc. New York. [Ref.]
- Rich ML, Ritterhoff RJ, Hoffman RJ. (1950) A fatal case of aplastic anaemia following chloramphenicol (chloromycetin) therapy. Ann Intern Med. 33: 1459-1467. [PubMed.]
- WallersteinR, Condit K, Kasper C, Brown J, et al. (1969) State Wide Study of chloramphenicol Therapy and Fatal Aplastic Anaemia. JAMA. 298: 2045-2050. [PubMed.]
- Rosenthal R, Blackman A. (1965) Bone marrow hypoplasia following use of chloramphenicol eye drops. JAMA. 191: 136-137. [PubMed.]
- Abrams SM, Gegnan TJ, Vinciguerra V. (1980) Marrow aplasia following topical application of chloramphenicol eye ointment. Arch Intern Med. 140: 576-577. [PubMed.]
- Fraunfelder FT, Bagby GC, Kelly DJ. (1982) Fatal aplastic anaemia following topical administration of ophthalmic chloramphenicol. Am J Ophthalmol. 93: 356-360. [PubMed.]
- Fraunfelder FW, Fraunfelder FT. (2007) Scientific challenges in post-marketing surveillance of ocular adverse drug reactions. Am J Ophthalmol. 143: 145-149. [PubMed.]
- Fraunfelder FT, Morgan RL, Yunis AA. (1993) Blood dyscrasia and topical ophthalmic chloramphenicol. Am J Ophthalmol. 115(6): 812-813. [PubMed.]
- Fraunfelder FW, Fraundfelder FT. (2013) Restricting topical ocular chloramphenicol eye drop use in the United States. Did we overreact? Am J Ophthalmol. 156(3): 420-422. [PubMed.]
- Besamusca and Bastiensen. (1986) Blood dyscrasias and topically applied chloramphenicol in ophthalmology. Doc Ophthalmol. 64: 87-95. [PubMed.]
- Wilholm B-E, Parsell Kelly J, Kaufman D, Issaragrisil S, et al. (1998) Relation of aplastic anaemia to use of chloramphenicol eye drops in two international case control studies. BMJ. 316: 666. [Ref.]
- Isenberg SJ. (2003) The fall and rise of chloramphenicol. J AAPOS. 7(5): 307-308. [PubMed.]
- McGhee CNJ, Anastas CN. (1996) Widespread ocular use of topical chloramphenicol: is there justifiable concern regarding idiosyncratic aplastic anaemia? Br J Ophthalmol. 80: 182-184. [PubMed.]
- (2014) New Zealand Medicines and Medical Devices Safety Authority. [Ref.]
- (2014) New Zealand Formulary. [Ref.]
- Robaei D, Naunton M, Watson S. (2015) Seeing red: over-the-counter chloramphenicol. Clin Exp Ophthalmol. 43: 99-100. [PubMed.]
- British National Formulary. (1998) British Medical Association and Royal Pharmaceutical Society of Great Britain. 35: 450. [Ref.]