>Corresponding Author : Gian Maria Pacifici
>Article Type : Review Article
>Volume : 2 | Issue : 1
>Received Date : 05 May, 2022
>Accepted Date : 15 May, 2022
>Published Date : 18 May, 2022
>DOI : https://doi.org/10.54289/JCTRE2200102
>Citation : Pacifici GM. (2022) Clinical Pharmacology of Cefotaxime. J Clin Trials Res Ethics 2(1): doi https://doi.org/10.54289/JCTRE2200102
>Copyright : © 2021 Pacifici GM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Review Article | Open Access
Associate Professor of Pharmacology via Sant’Andrea 32, 56127 Pisa, Italy
*Corresponding author: Gian Maria Pacifici, Associate Professor of Pharmacology via Sant’Andrea 32, 56127 Pisa, Italy
Abstract
Cefotaxime is a third-generation cephalosporin and is resistant to many narrow-spectrum β-lactamases and is active against most gram-positive and gram-negative aerobic bacteria. The efficacy and safety of cefotaxime have been reported and cefotaxime is metabolized into desacetylcefotaxime and desacetylcefotaxime-lactone. Cefotaxime diffuses into human brain and lung in significant amounts. The pharmacokinetics of cefotaxime have been studied in healthy volunteers. The mean peak concentration of cefotaxime is 11.7 and 20.5 µg/ml following an intravenous cefotaxime dose of 500 and 1,000 mg, respectively, and the elimination half-life of cefotaxime is about 1 hour. The prophylaxis, treatment, and trials with cefotaxime have been studied. The penetration of cefotaxime into the cerebrospinal fluid has been investigated. Following an intravenous administration of 2 grams of cefotaxime, the mean peak concentration of cefotaxime in the cerebrospinal fluid is 0.61 µg/ml and cefotaxime is eliminated from the cerebrospinal fluid with a mean half-life of 12.6 hours. The mean peak concentration of desacetylcefotaxime in the cerebrospinal fluid and the mean elimination half-life of desacetylcefotaxime from the cerebrospinal fluid are 0.18 µg/ml and 15.5 hours, respectively, and cefotaxime treats bacterial meningitis. Cefotaxime may become resistant to bacteria, freely crosses the human placenta, and poorly migrates into the breast-milk. The aim of this study is to review cefotaxime efficacy and safety, metabolism, diffusion into human brain and lung, pharmacokinetics, prophylaxis, treatment, trials, cefotaxime penetration into the cerebrospinal fluid, treatment of bacterial meningitis, transfer across the human placenta, migration into the breast-milk, and cefotaxime resistance to bacteria.
Keywords: Breast-Milk; Cefotaxime; Cerebrospinal-Fluid; Desacetylcefotaxime; Efficacy-Safety; Meningitis; Metabolism; Pharmacokinetics; Placenta; Prophylaxis; Resistance; Treatment; Trials
Abbreviations: CSF: Cerebrospinal Fluid, AUC: Area Under the Concentration-Time Curve, Tmax: Time to Reach the Peak Concentration
Introduction
Cefotaxime is a third-generation cephalosporin and is resistant to many narrow-spectrum β-lactamases and has good activity against most gram-positive and gram-negative aerobic bacteria. Cefotaxime has an elimination half-life in plasma of about 1 hour and should be administered 4 times-daily of thrice-daily for treatment of serious infections. Cefotaxime is metabolized in-vivo into desacetylcefotaxime, which is less active than is the parent compound.
Concentrations achieved in the cerebrospinal fluid are adequate for treatment of meningitis caused by Haemophilus influenzae, penicillin-sensitive Streptococcus pneumoniae, and Neisseria meningitidis [1].
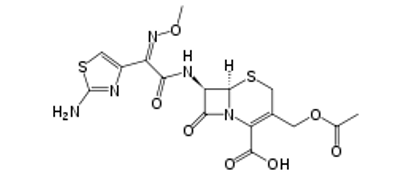
Cefotaxime molecular structure (molecular weight = 455.47 grams/mole)
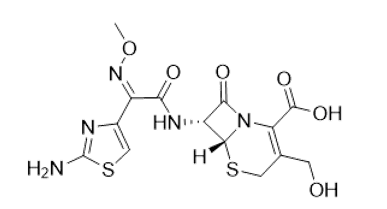
Desacetylcefotaxime molecular structure (molecular weight = 413.4 grams/mole)
Literature search
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “cefotaxime efficacy safety”, “cefotaxime metabolism”, “cefotaxime tissue concentration”, “cefotaxime pharmacokinetics”, “cefotaxime prophylaxis” “cefotaxime treatment”, “cefotaxime trials”, cefotaxime CSF”, “cefotaxime meningitis”, “cefotaxime resistance”, “cefotaxime placental transfer”, and “cefotaxime breast-milk”. In addition, the book “The pharmacological basis of therapeutics” [1] has been consulted.
Results
Efficacy and safety of cefotaxime
Cefotaxime-sulbactam administered thrice-daily for up to 7 days was effective and safe in treatment of respiratory-tract infections in children [2]. Cefotaxime in combination with metronidazole is a safe and highly effective treatment of brain abscess [3]. Cefotaxime, administered intravenously at a dose of 1 gram thrice-daily or at a dose of 2 grams thrice-daily, is efficacy and safe in treating pneumonia [4]. Cefotaxime was administered intravenously at a dose of 1 gram thrice-daily or at a dose 2 grams thrice-daily and cefotaxime was efficacy and safe in treating patients infected with HIV and improved quality of life by reducing the length of hospital stay [5]. Cefotaxime effectively and safety treated serious bacterial infections in children [6]. Cefotaxime is efficacy and safe in treatment of serious bacterial infections in children [7]. Cefotaxime effectively and safety treated lower respiratory-tract infection, meningitis, and septicaemia in paediatric patients [8].
Metabolism of cefotaxime
14C-cefotaxime was administered to humans, rats, and rabbits and 80% of radioactivity was recovered in the urine and desacetylcefotaxime is the major metabolite found in plasma and it was excreted in the urine. The metabolic pathway follows the following route: cefotaxime-desacetylcefotaxime, desacetylcefotaxime-lactone and 2 unidentified metabolites [9]. The metabolism of cefotaxime was studied in rat, dog, and man and following intramuscular administration of cefotaxime this drug was recovered in the urine in similar amounts in animals and man. Desacetylcefotaxime was the major metabolite found in plasma. In-vitro studies revealed the following metabolite route: desacetylcefotaxime, desacetylcefotaxime-lactone and two unidentified metabolites, and cefotaxime and all metabolites were excreted in the urine [10]. A single dose of cefotaxime was administered to patients with renal failure. The main metabolite found in plasma was desacetylcefotaxime; this metabolite, desacetylcefotaxime-lactone and two unidentified metabolites were excreted in the urine. In-vitro studies revealed that the two undefined metabolites are formed by the degradation of desacetylcefotaxime-lactone [11].
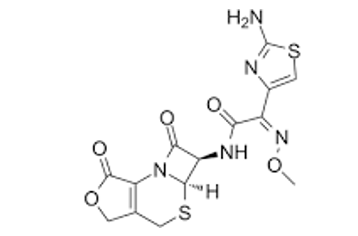
Desacetylcefotaxime-lactone molecular structure (molecular weight = 395.41 grams/mole)
Diffusion of cefotaxime in human brain and lung
Cefotaxime was administered intravenously at a dose of 3 grams thrice-daily to 8 patients and the concentration of cefotaxime and desacetylcefotaxime were measured in brain abscesses. The brain abscess concentrations of cefotaxime and desacetylcefotaxime were 1.9+1.7 and 4.0+2.2 µg/ml, respectively. The simultaneous concentrations of cefotaxime and desacetylcefotaxime in plasma were 2.0+1.0 and 3.9+1.8 µg/ml, respectively. With increasing time following cefotaxime dosing there was a significant increase in the brain abscess to plasma concentration ratio of desacetylcefotaxime [12]. Cefotaxime was administered intravenously at a dose of 4 grams thrice-daily to a patient with traumatic brain injury and the maximal brain extracellular fluid concentration of cefotaxime was 11.4 µg/ml. This limited brain distribution of cefotaxime may be explained by the blood-brain barrier which is known to express efflux transporters like P-glycoprotein or multidrug resistant-associated protein [13]. Cefotaxime was administered intravenously at a dose of 2 grams to 34 patients undergoing lung surgery. Sixty min after injection, ceftizoxime concentrations in the lung tissue ranged between 7 and 15 µg/gram (mean 11) and declined slowly thereafter. After 4 hours cefotaxime administration, cefotaxime concentration ranged between 2 and 4 µg/gram, and at 7 hour after dosing, cefotaxime concentration was 1 µg/gram [14].
Pharmacokinetics of cefotaxime
Fu et al. [15] studied the pharmacokinetics of cefotaxime in 24 male healthy human volunteers, aged 21 to 33 years and weighing 78+4 kg, and cefotaxime was administered intramuscularly or intravenously. Seven subjects received cefotaxime intramuscularly at a dose of 500 mg and 7 subjects received 500 mg of cefotaxime intravenously and 10 subjects received cefotaxime intravenously at a dose of 1,000 mg.
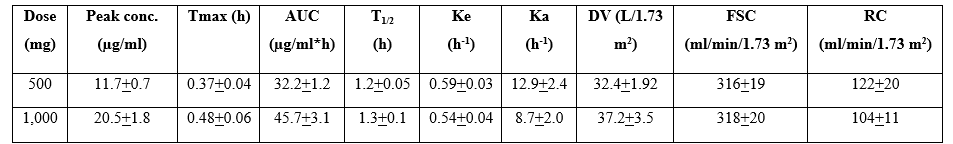
Table 1. Pharmacokinetic parameters of cefotaxime which have been obtained following cefotaxime administered intravenously at a dose of 500 or 1,000 mg. Values are the mean+SD, by Fu et al. [15].
Tmax = time to reach the peak concentration. AUC = area under the concentration-time curve. T1/2 = elimination half-life. Ka = absorption-rate constant. Ke = elimination-rate constant. DV = distribution volume. FSC = fractional serum clearance. RC = renal clearance.
This table shows that cefotaxime is rapidly absorbed as Tmax is less than 0.5 hours and the absorption-rate constant is about 10 h-1, cefotaxime is rapidly eliminated as the elimination half-life is about 1 hour and the elimination-rate constant is about 0.5 h-1. All pharmacokinetic parameters are not significantly different according to the 2 cefotaxime doses and there is a limited interindividual variability in the pharmacokinetic parameters.
Prophylaxis with cefotaxime for prevention of infections during surgery
Cefotaxime-heparin effectively reduced catheter-related bloodstream infections caused by gram-positive cocci including methicillin-susceptible Staphylococcus aureus [16]. Prophylaxis with cefotaxime reduced wound infections in patients undergoing abdominal surgery [17]. Single-dose of 1 gram of cefotaxime prevents infections in patients undergoing hysterectomy, Caesarean sections, bone and joint procedures, upper gastrointestinal surgery, biliary tract procedures, transurethral resections, and open urologic procedures [18]. A single-dose of 1 gram or 2 grams of cefotaxime, administered 30 minutes prior to surgery, is an effective prophylaxis for infection following gastrointestinal, biliary, obstetric, gynaecologic, and genital-urinary surgery [19]. Short-term cefotaxime prophylaxis prevents infections in patients undergoing lower limb amputations [20]. Prophylaxis, with a single-dose of cefotaxime, is more effective than 4 doses of cefoxitin and cefazolin in preventing infections in women undergoing gynaecologic surgery [21]. Prophylaxis with cefotaxime prevents infections in patients undergoing surgery [22]. Prophylaxis with a single-dose of cefotaxime, administered at a dose of 1 gram or 2 grams daily, effectively prevents infection in patients undergoing surgery [23]. Short-term prophylaxis with cefotaxime is effective as long-term prophylaxis with penicillin and streptomycin in preventing infection in patients undergoing colorectal surgery [24]. Prophylaxis with cefotaxime prevents infections in patients undergoing prostatic surgery [25]. Short-term prophylaxis with cefotaxime significantly reduced the rate of urinary infection after transurethral prostatectomy [26].
Treatment of bacterial infections with cefotaxime
Cefotaxime sodium is more effective than netilmicin in treatment of patients with septic shock [27]. Amoxicillin-clavulanic acid is effective as cefotaxime in treatment of bacterial infections in cirrhotic patients [28]. Cefotaxime is an effective and well-tolerated agent in the treatment of serious urologic infections [29]. Empirical treatment with cefotaxime is effective in 81% of patients with bacterial peritonitis [30]. Cefotaxime effectively treats lower respiratory-tract infection during surgery [31]. Cefotaxime is the agent of choice for the empiric treatment of bacterial peritonitis [32]. Cefotaxime is of great clinical value in treatment of life-threatening where other therapies failed [33]. Cefotaxime, administered at a dose of 2 grams thrice-daily, treats serious chest infections [34]. Cefotaxime is clinically effective in treatment of serious infection and does not induce adverse-effects [35]. Cefotaxime is an effective antibacterial agent for the treatment of mild to moderate infections of infections of the central nervous system [36]. Cefotaxime treats infections caused by Staphylococcus aureus, Proteus mirabilis, Escherichia coli, or Pseudomonas aeruginosa [37]. Ciprofloxacin, taken intravenously twice-daily, is effective as cefotaxime, administered intravenously thrice-daily, in treatment of skin and skin structure infections [38]. Cefotaxime, administered intravenously at a dose of 1 gram or 2 grams twice-daily or thrice-daily, treats bronchial and lung tissue infections [39].
Trials with cefotaxime
Cefepime, administered intravenously at a dose of 2 grams twice-daily, was at least effective and well tolerated as cefotaxime, administered intravenously at a dose of 2 grams thrice-daily, in the treatment of bacterial pneumonia in HIV-infected patients [40]. A trial demonstrated that cefotaxime is the first-choice antibiotic in the therapy of spontaneous bacterial peritonitis in cirrhotic patients [41]. Cefotaxime significantly reduced the incidence of early postoperative bacteriuria without causing any significant adverse-effects [42]. Oral ciprofloxacin, administered intravenously at a dose of 750 mg twice-daily, was safe and effective as parenteral cefotaxime, administered at a dose of 2 grams thrice-daily, in treatment of difficult infections of the skin and skin structure [43]. A trial demonstrated that cefotaxime treats patients with infections due to Streptococcus pneumoniae, Haemophilus influenzae, Streptococcus pyogenes, Staphylococcus aureus, Escherichia coli, Proteus, or Klebsiella species [44].
Cefotaxime was significantly more effective (P-value < 0.01) than cefazolin in eradicating Escherichia coli or Proteus mirabilis from the urinary-tract [45]. Cefotaxime is more effective and less toxic than nafcillin plus tobramycin in treatment of patients with serious bacterial infections [46].
Penetration of cefotaxime into the cerebrospinal fluid (CSF)
Nau et al. [47] investigated the penetration of cefotaxime and desacetylcefotaxime into the CSF of 6 patients with uninflamed meninges and a single dose of 2 grams of cefotaxime was intravenously infused.
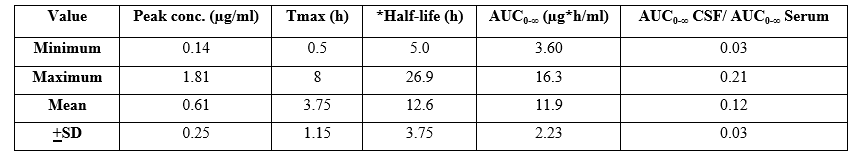
Table 2. Single-dose pharmacokinetics of cefotaxime in cerebrospinal fluid (CSF). Values are the minimum, maximum, mean, and +SD, by Nau et al. [47].
Tmax = time to reach the peak concentration. *Elimination half-life. AUC = area under the concentration-time curve.
The mean Tmax is 3.75 hours suggesting that cefotaxime rapidly penetrates into the CSF. The elimination half-life of cefotaxime in serum is 2.19+0.44 hours, thus the half-life of cefotaxime is longer in CSF than in serum. The cefotaxime mean AUC in CSF to AUC in serum ratio is 0.12 suggesting that cefotaxime resides in serum in major amounts than in CSF. In addition, there is a remarkable interindividual variability of the pharmacokinetic parameters.
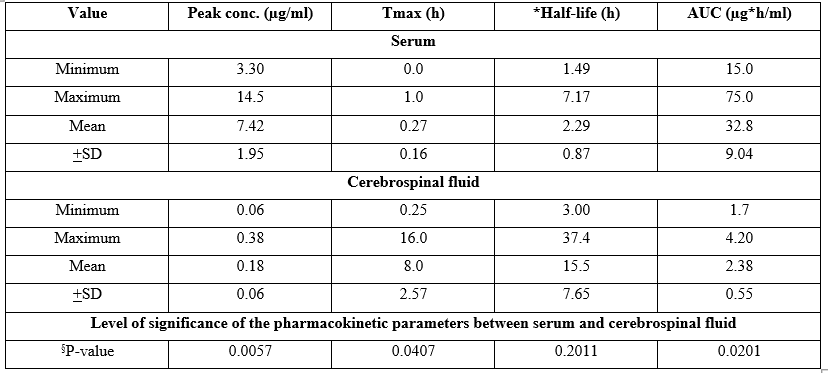
Table 3. Single-dose pharmacokinetics of desacetylcefotaxime in serum and in cerebrospinal fluid. Values are the minimum, maximum, mean, and +SD, by Nau et al. [47].
§Unpaired t test.
Tmax = time to reach the peak concentration. *Elimination half-life. AUC = area under the concentration-time curve.
This table shows that the peak concentration of desacetylcefotaxime is higher in serum than in the CSF, Tmax is shorter in serum than in the CSF, and desacetylcefotaxime elimination half-life is not different in serum and in the CSF. The lack of difference of desacetylcefotaxime half-life between serum and CSF is due by wide variability of this parameter in the cerebrospinal fluid. The AUC value is higher in serum than in the CSF suggesting that desacetylcefotaxime preferentially resides in serum. In addition, there is a remarkable interindividual variability of desacetylcefotaxime in the cerebrospinal fluid.
The penetration of cefotaxime into the CSF was studied in 31 patients with pneumococcal meningitis, aged 61 years (range, 52 to 69), and cefotaxime was intravenously infused at a dose of 200 mg/kg (4.8 to 19.3). Median (inter quartile range) cefotaxime CSF concentration was 10.3 µg/ml (range, 4.8-19.3). This concentration is higher the minimum inhibitory concentration (0.25 µg/ml) of the pathogen causing the meningitis. Cefotaxime penetrates into the CSF is significant amounts and treated the meningitis caused by Pneumococcus meningitis in all patients [48]. The concentration of cefotaxime was measured in the cerebrospinal fluid of 33 children with meningitis caused by Streptococcus pneumoniae, Haemophilus influenzae type b, or by Neisseria meningitidis and cefotaxime was administered intravenously at a dose of 50 mg/kg 4 times-daily. The lowest cefotaxime concentration in the cerebrospinal fluid was 0.45 µg/ml. The minimum inhibitory concentration of the organisms causing the meningitis ranged from 0.002 to 0.01 µg/ml. The cefotaxime concentration in the cerebrospinal fluid was higher than the minimum inhibitory concentration of the organisms causing the meningitis and the meningitis was cured in all children [49]. Cefotaxime sodium was administered to 10 patients with the meningitis caused by Pseudomonas aeruginosa and the trough cefotaxime concentration into the CSF ranged from 5.6 to 44.3 µg/ml and that of desacetylcefotaxime ranged from 3.7 to 44.0 µg/ml. These concentrations are many-fold greater the minimum inhibitory concentration of Pseudomonas aeruginosa (≤ 0.5 µg/ml) and the meningitis was cured in all patients [50].
Treatment of bacterial meningitis with cefotaxime
Ten patients had the meningitis caused by Streptococcus pneumoniae and were treated with cefotaxime given intravenously at a dose of 300 mg/kg (maximum dose, 24 grams daily). The cefotaxime concentration in the cerebrospinal fluid ranged from 1 to 4 µg/ml and this concentration was higher the minimum inhibitory concentration (≤ 0.5 µg/ml) of Streptococcus pneumoniae and the meningitis was cured in all patients [51]. Fifty children with bacterial meningitis caused by Haemophilus influenzae received cefotaxime intravenously at a dose of 50 mg/kg 4 times-daily and the duration of therapy, 11.1+2.4 days (range, 10 to 21 days). No adverse-effects were observed and cefotaxime cured the meningitis in all children [52]. Thirty-eight infants and children aged 6 weeks to 16 years with bacterial meningitis received cefotaxime intravenously at a dose of 200 mg/kg for 4 to 7 days. The mean trough concentration of cefotaxime in the cerebrospinal fluid was 2.7 µg/ml, the cerebrospinal fluid was sterilized after 24 hours of treatment, and the meningitis was cured in all infants and children [53]. Thirty children with meningitis caused by Streptococcus pneumoniae, Neisseria meningitidis, group B streptococcus, or Salmonella enteritidis received cefotaxime intravenously at a dose of 50 mg/kg 4 times-daily. The cerebrospinal fluid concentrations of cefotaxime and desacetylcefotaxime, measured one hour after drug administration, were 3.72+5.57 µg/ml and 4.35+7.12 µg/ml, the cerebrospinal fluid was sterilized, and cefotaxime cured the meningitis in all children [54]. Thirty-one children with bacterial meningitis were treated with cefotaxime intravenously at a dose of 100 mg/kg thrice-daily. The cerebrospinal fluid was sterilized after 2 days of treatment and the meningitis was cured in all children [55].
Resistance of bacteria to cefotaxime
Haemophilus influenzae may become resistant to cefotaxime and the resistance was caused by alterations of L389F and Y557H genes [56]. Of 160 isolates of Escherichia coli, 98.3% were cefotaxime-resistant, and the genes causing the resistance were blaCTX-M-65, blaCTX-M-55 and blaCTX-M-3 [57]. The overall prevalence of cefotaxime-resistant bacteria was 15.8% varied between farms, and ranged from 5.2% to 100%. The resistance was caused by a modification of the extended spectrum β-lactamase genes [58]. One-hundred-three elderly patients were infected by gram-negative bacteria and 26 patients (18.2%) were resistant to cefotaxime. The resistance was due to alteration of AmpC β-lactamase and CMY-2 β-lactamase genes [59]. The resistance of Citrobacter freundii was caused by an increase of the minimum inhibitory concentration from 0.12 to 256 µg/ml [60].
Transfer of cefotaxime across the human placenta
In literature there is only one study on the placental transfer of cefotaxime and it has been reported by Brown et al. [61].
mean cefotaxime concentration was 18.04+3.37 µg/ml in the umbilical vein plasma and 21.02+17.8 µg/ml in the maternal plasma shortly after administration. Thus cefotaxime freely crosses the human placenta.
Migration of cefotaxime into the breast-milk
A single intravenous dose of 1 gram of cefotaxime was administered to 42 lactating women and the mean peak concentration in the breast-milk was 0.32+0.09 µg/ml [62]. A singly intravenous dose of 1 gram of cefotaxime was administered to 12 lactating women and the mean peak concentration of cefotaxime was 0.32 µg/ml [63]. Cefotaxime was administered intravenously at a dose of 1 gram to 12 lactating women and the mean peak concentration of cefotaxime in the beast-milk was 0.35+0.09 µg/ml (range, 0.25 to 0.52) 2 to 3 hours after administration [64]. These results are consistent with the view that cefotaxime poorly migrates into the breast-milk.
Discussion
Cefotaxime is a third-generation cephalosporin and is resistant to many narrow-spectrum β-lactamases and is active against most gram-positive and gram-negative aerobic bacteria [1]. The efficacy and safety of cefotaxime has been reported [2-8]. Cefotaxime-sulbactam administered thrice-daily for 7 days is effective and safe in treatment of children with respiratory-tract infections [2], cefotaxime plus metronidazole effectively treats brain abscess [3], cefotaxime, administered intravenously at a dose of 1 gram thrice-daily or at a dose of 2 grams thrice-daily, effectively treats pneumonia [4], cefotaxime, administered intravenously at a dose of 1 gram thrice-daily or at a dose of 2 grams thrice-daily, effectively and safety treats patients with HIV and improved the quality of life by reducing the hospital stay [5], cefotaxime effectively and safety treats serious bacterial infections in children [6,7], and cefotaxime effectively and safety treats lower respiratory-tract infections, meningitis, and septicaemia in paediatric patients [8]. The metabolism of cefotaxime has been studied [9-11]. Cefotaxime is metabolized into desacetylcefotaxime, desacetylcefotaxime-lactone and into 2 unidentified metabolites and all metabolites are eliminated in the urine. Cefotaxime diffuses into the human brain and lung is significant amounts [12-14]. The pharmacokinetics of cefotaxime have been studied in male healthy volunteers following the intravenous administration of cefotaxime at a dose of 500 mg or 1,000 mg [15]. The mean peak concentration of cefotaxime is 11.7 and 20.5 µg/ml, following a cefotaxime dose of 500 and 1,000 mg, respectively, and the elimination half-life of cefotaxime is about 1 hour. The prophylaxis with cefotaxime has been extensively studied [16-26]. Cefotaxime-heparin reduces the catheter-related infections caused by gram-positive cocci including methicillin-susceptible Staphylococcus aureus [16]. Prophylaxis with cefotaxime reduces wound infections in patients undergoing abdominal surgery [17]. A single-dose of 1 gram cefotaxime prevents infections in patients undergoing hysterectomy, Caesarean section, bone, joint, biliary, and transurethral procedures [18]. A single-dose of 1 gram or 2 grams of cefotaxime prevents infection in patients undergoing gastrointestinal, biliary, obstetrics, gynaecologic, and genital-urinary surgery [19]. A short-term cefotaxime prevents infections in patients undergoing limb amputations [20]. Prophylaxis with a single dose of cefotaxime is more effective than 4 doses of cefoxitin and cefazolin in preventing infections in women undergoing gynaecologic surgery [21]. Prophylaxis with cefoxitin prevents infections in patients undergoing surgery [22]. A single-dose of 1 gram or 2 grams of cefotaxime prevents infections in patients undergoing surgery [23]. Prophylaxis with short-term cefotaxime is effective as long-term prophylaxis with penicillin and streptomycin in preventing infections in patients undergoing colorectal surgery [24]. Prophylaxis with cefotaxime prevents infections in patients undergoing prostatic surgery [25] and reduces the rate of urinary infections after transurethral prostatectomy [26]. The treatment of bacterial infections with cefotaxime has been extensively reported [27-39]. Cefotaxime sodium is more effective than netilmicin in treatment of patients with septic shock [27]. Amoxicillin/clavulanic acid is effective as cefotaxime in treatment of bacterial infections [28]. Cefotaxime effectively treats serious urologic infections [29], effectively treats bacterial peritonitis [30, 32], effectively treats lower respiratory-tract and cefotaxime effectively treats serious infections [35], effectively treats mild to moderate infections of the central nervous [36], and effectively treats infections caused by Staphylococcus aureus, Proteus mirabilis, Escherichia coli, or by Pseudomonas aeruginosa [37]. Ciprofloxacin, administered intravenously twice-daily, is effective as cefotaxime, administered intravenously thrice-daily, in treatment of skin and skin structure infections [38]. Cefotaxime, administered at a doe of 1 gram or 2 grams twice-daily or thrice-daily, treats bronchial and lung infections [39]. The trials with cefotaxime have been reported [40-46]. Cefepime, administered intravenously at a dose of 2 grams twice-daily, is effective and well tolerated as cefotaxime, administered intravenously at a dose of 2 grams thrice-daily, in treatment of bacterial pneumonia in HIV-infected patients [40]. Cefotaxime effectively treats bacterial peritonitis in cirrhotic patients [41], and cefotaxime reduces the incidence of postoperative bacteriuria [42]. Oral ciprofloxacin, administered intravenously at a dose of 750 mg thrice-daily, is effective and safe as parenteral cefotaxime, administered at a dose of 2 grams thrice-daily, in treatment of skin and skin structure infections [43]. Cefotaxime treats patients with infections due to Streptococcus pneumoniae, Haemophilus influenzae, Streptococcus pyogenes, Staphylococcus aureus, Escherichia coli, or Klebsiella species [44]. Cefotaxime is more effective than cefazolin in eradicating Escherichia coli and Proteus mirabilis from urinary-tract [45], and cefotaxime is more effective than nafcillin plus tobramycin in treatment of serious bacterial infections [46]. The penetration of cefotaxime into the cerebrospinal fluid has been studied [47-50]. Following an intravenous administration of cefotaxime at a dose of 2 grams, the mean peak concentration of cefotaxime in the cerebrospinal fluid is 0.61 µg/ml and the mean elimination half-life of cefotaxime from the cerebrospinal fluid is 12.6 hours. The mean area under the concentration-time curve (AUC) of cefotaxime in the cerebrospinal fluid to AUC of cefotaxime in serum ratio is 0.12. The mean peak concentration of desacetylcefotaxime in serum and in the cerebrospinal fluid is 7.42 and 0.18 µg/ml, respectively. Desacetylcefotaxime is eliminated from the serum and from the cerebrospinal fluid with a mean half-life of 2.29 and 15.5 hours, respectively [47]. The penetration of cefotaxime into the cerebrospinal fluid was studied in patients with pneumococcal meningitis and cefotaxime was administered intravenously at a mean dose of 200 mg/kg and the mean cefotaxime concentration in the cerebrospinal fluid is 10.3 µg/ml. This concentration is higher the minimum inhibitory concentration (≤ 0.25 µg/ml) of Pneumococcus meningitis and meningitis was cured in all patients [48]. Cefotaxime was administered intravenously at a dose of 50 mg/kg 4 times-daily to children with meningitis caused by Streptococcus pneumoniae, Haemophilus influenzae type b, or by Neisseria meningitidis. The lowest cefotaxime concentration in the cerebrospinal fluid is 0.45 µg/ml and this concentration is higher the minimum inhibitory concentration (range, 0.002 to 0.01 µg/ml) of the organisms causing the meningitis and the meningitis was cured in all children [49]. Cefotaxime sodium was administered intravenously to patients with meningitis due to Pseudomonas aeruginosa and the concentration of cefotaxime in the cerebrospinal fluid ranged from 5.6 to 44.3 µg/ml. This concentration is many-fold higher the minimum inhibitory concentration of the infective pathogen and the meningitis was cured in all patients [50]. The treatment of bacterial meningitis has been studied [51-55]. Cefotaxime was administered at a mean dose of 300 mg/kg (maximum dose, 24 grams daily) to patients with meningitis caused by Streptococcus pneumoniae. The cefotaxime concentration in the cerebrospinal fluid ranged from 1 to 4 µg/ml and this concentration is higher the minimum inhibitory concentration (≤ 0.5 µg/ml) of the infective organism and the meningitis was cured in all patients [51]. Fifty children had the meningitis caused by Haemophilus influenzae and were treated with cefotaxime at a dose of 50 mg/kg 4 times-daily and the meningitis was cured in all children [52]. Thirty-eight infants and children with bacterial meningitis were treated with cefotaxime at a dose of 200 mg/kg for 4 to 7 days. The mean trough concentration of cefotaxime in the cerebrospinal fluid 2.7 µg/ml and the meningitis was cured in all infants and children [53]. Thirty children had the meningitis caused by Streptococcus pneumoniae, Neisseria meningitidis, group B Streptococcus or by Salmonella enterica and were treated with cefotaxime intravenously at a dose of 50 mg/kg 4 times-daily. The mean concentration of cefotaxime and desacetylcefotaxime were 3.72 and 4.35 µg/ml, respectively, and the cerebrospinal fluid was sterilized [54]. Thirty-one children with bacterial meningitis were treated with cefotaxime intravenously at a dose 100 mg/kg thrice-daily and the cerebrospinal fluid was satirized after 2 days of treatment [55]. Some bacteria may become resistant to cefotaxime [56-60]. The resistance of Haemophilus influenzae to cefotaxime is caused by alterations of L289F and Y557H genes [56], the resistance of Escherichia coli to cefotaxime is caused by resistance genes blaCTX-M-65, blaCTX-M-55, and blaCTX-M-3 [57], the resistance of bacteria to cefotaxime is due to modification of the extended spectrum β-lactamase genes [58], and the resistance of gram-negative bacteria is caused by alteration of AmpC β-lactamase and CMY-2 β-lactamase genes [60]. Cefotaxime freely crosses the human placenta [61]. The migration of cefotaxime into the breast-milk has been reported in 3 studies [62-64] and the peak concentration of cefotaxime in the breast-milk is less than 1 µg/ml indicating that cefotaxime poorly migrates into the beast-milk.
In conclusion, cefotaxime is a third-generation cephalosporin and is resistant to many narrow-spectrum β-lactamases and is active against most gram-positive and gram-negative aerobic bacteria. The efficacy and safety of cefotaxime has been reported and cefotaxime is metabolized into desacetylcefotaxime, desacetylcefotaxime-lactone and 2 unidentified metabolites. Cefotaxime diffuses in human brain and lung is significant amounts. The pharmacokinetics of cefotaxime have been described. Following the intravenous administration of cefotaxime at a dose of 500 or 1,000 mg, the cefotaxime mean peak concentration is 11.7 and 20.5 µg/ml, respectively, and cefotaxime elimination half-life is about 1 hour. The prophylaxis, treatment, and trials with cefotaxime have been extensively studied. Cefotaxime penetrates into the cerebrospinal fluid is significant amounts and treats bacterial meningitis. Some bacterial may become resistant to cefotaxime and cefotaxime freely crosses the human placenta and poorly migrates into the breast-milk. The aim of this study is to review the clinical pharmacology of cefotaxime.
Conflict of interests
The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria.
This article is a review and drugs have not been administered to men or animals.
Acknowledgments
The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.
References
- MacDougal C. (2018) “Penicillins, Cephalosporin, and Other β-Lactam Antibiotics”. In The Goodman & Gilman’s. The Pharmacological Basis of the Therapeutics, Brunton Hilal-dandan LL, Knollmann BC, editors. Mc Graw Hill, 13th Edition, USA, New York. 1023-1038. [Ref.]
- Pareek A, Kulkarni M, Daga S, Deshpande A, Chandurkar N. (2008) Comparative evaluation of efficacy and safety of cefotaxime-sulbactam with amoxicillin-clavulanic acid in children with lower respiratory tract infections. Expert Opin Pharmacother. 9(16): 2751-2757. [PubMed.]
- Jansson A-K, Enblad P, Sjölin J. (2004) Efficacy and safety of cefotaxime in combination with metronidazole for empirical treatment of brain abscess in clinical practice: a retrospective study of 66 consecutive cases. Eur J Clin Microbiol Infect Dis. 23(1): 7-14. [PubMed.]
- Morales JO, Snead H. (1994) Efficacy and safety of intravenous cefotaxime for treating pneumonia in outpatients. Am J Med. 97(2A): 28-33. [PubMed.]
- Morales JO, Von Behren L. (1994) Secondary bacterial infections in HIV-infected patients: an alternative ambulatory outpatient treatment utilizing intravenous cefotaxime. Am J Med. 97(2A): 9-13. [PubMed.]
- Jacobs RF, Darville T, Parks JA, Enderlin G. (1992) Safety profile and efficacy of cefotaxime for the treatment of hospitalized children. Clin Infect Dis. 14(1): 56-65. [PubMed.]
- Jacobs RF. (1991) Efficacy and safety of cefotaxime in the management of pediatric infections. Infection. 19(Suppl 6): S330-S336. [PubMed.]
- Young JP, Husson JM, Bruch K, Blomer RJ, Savopoulos C. (1980) The evaluation of efficacy and safety of cefotaxime: a review of 2500 cases. J Antimicrob Chemother. 6(Suppl A): 293-300. [PubMed.]
- Lassman HB, Coombes JD. (1984) Metabolism of cefotaxime: a review. Diagn Microbiol Infect Dis. 2(Suppl 3): 3S-12S. [PubMed.]
- Chamberlain J, Coombes JD, Dell D, Fromson JM, Ings RJ, et al. (1980) Metabolism of cefotaxime in animals and man. J Antimicrob Chemother. 6(Suppl A): 69-78. [PubMed.]
- Reeves DS, White LO, Holt HA, Bahari D, Bywater MJ, et al. (1980) Human metabolism of cefotaxime. J Antimicrob Chemother. 6(Suppl A): 93-101. [Ref.]
- Sjölin J, Eriksson N, Arneborn P, Cars O. (1991) Penetration of cefotaxime and desacetylcefotaxime into brain abscesses in humans. Antimicrob Agents Chemother. 35(12): 2606-2610. [Ref.]
- Frasca D, Dahyot-Fizelier C, Couet W, Debaene B, Mimoz O, et al. (2012) Brain microdialysis distribution study of cefotaxime in a patient with traumatic brain injury. Br J Anasth. 109(5): 830-831. [Ref.]
- Adam D, König J. (1983) Tissue concentration of cefotaxime in the lung. Arzneimittelforschung. 33(3): 427-429. [PubMed.]
- Fu KP, Aswapokee P, Ho I, Matthijssen C, Neu HC. (1979) Pharmacokinetics of cefotaxime. Antimicrob Agents Chemother. 16(5): 592-597. [Ref.]
- Saxena AK, Panhotra BR, Al-hafiz AA, Sundaram DS, Abu-Oyun B, et al. (2012) Cefotaxime-heparin lock prophylaxis against hemodialysis catheter-related sepsis among Staphylococcus aureus nasal carriers. Saudi J Kidney Dis Transpl. 23(4): 743-754. [PubMed.]
- Sader HS, Jones RN. (1992) Cefotaxime is extensively used for surgical prophylaxis. Am J Surg. 164(4A Suppl): 28S-38S. [PubMed.]
- Jones RN. (1990) Review of cefotaxime sodium for surgical prophylaxis. A model for the evolution toward single-dose or short-course cost-effective regimens. Diagn Microbiol Infect Dis. 13(4): 317-327. [Ref.]
- Gentry LO. (1990) Cefotaxime and prophylaxis. New approaches with a proven agent. Am J Med. 88(4A): 32S-37S. [PubMed.]
- Norlin R, Frydén A, Nilsson L, S Anséhn S. (1990) Short-term cefotaxime prophylaxis reduces the failure rate in lower limb amputations. Acta Orthop Scand. 61(5): 460-462. [PubMed.]
- Campillo F, Rubio JM. (1992) Comparative study of single-dose cefotaxime and multiple doses of cefoxitin and cefazolin as prophylaxis in gynecologic surgery. Am J Surg. 164(Suppl 4 A): 12S-15S. [PubMed.]
- Wittmann DH. (1995) The role of cefotaxime in the treatment of surgical infections. Diagn Microbiol Infect Dis. 22(1-2): 173-182. [PubMed.]
- Wittmann DH, Jones RN, Malledant J, Privitera G. (1997) Cefotaxime in the treatment of prophylaxis of surgical infections. J Chemother. 9(Suppl 2): 19-33. [PubMed.]
- Lauridsen F, Bjoernsen K, Nielsen SA, Hansen OH. (1988) Short-term prophylaxis with cefotaxime in colorectal surgery. A prospective, randomized trial. Dis Colon Rectum. 31(1): 25-27. [PubMed.]
- Hargreave TB, Hindmarsh JR, Elton R, Chisholm GD, Gould JC. (1982) Short-term prophylaxis with cefotaxime for prostatic surgery. Br Med J (Clin Res Ed). 284(6321): 1008-1010. [Ref.]
- Botto H, Richard F, Mathieu F, Perreau AM, Camey M. (1984) Short-term prophylaxis with cefotaxime in prostatic surgery. J Antimicrob Chemother. 14(Suppl B): 231-235. [PubMed.]
- Pang S, Song Y, Yang J, Li S, He Y, et al. (2021) Efficacy of Etimicin Sulfate Combined with Cefotaxime Sodium in the Treatment of Patients with Septic Shock and Effect on Serum Inflammatory Factor Levels and Immune Function. Evid Based Complement Alternat Med. 8583118. [Ref.]
- Ricart E, Soriano G, Novella MT, Ortiz J, Sàbat M, et al. (2000) Amoxicillin-clavulanic acid versus cefotaxime in the therapy of bacterial infections in cirrhotic patients. J Hepatol. 32(4): 596-602. [PubMed.]
- Rasmussen D, Bremmelgaard A, Rasmussen F, Thorup J. (1986) Treatment of serious urological infections with cefotaxime compared to ampicillin plus netilmicin. Dan Med Bull. 33(1): 49-51. [PubMed.]
- Badawy AA, Zaher TI, Sharaf SM, Emara MH, Shaheen NE, et al. (2013) Effect of alternative antibiotics in treatment of cefotaxime resistant spontaneous bacterial peritonitis. World J Gastroenterol. 19(8): 1271-1277. [Ref.]
- Robertson MB, Korman TM, Dartnell JGA, Ioannides-Demos LL, Kirsa SW, et al. (2002) Ceftriaxone and cefotaxime use in Victorian hospitals. Med J Aust. 176(11): 524-529. [Ref.]
- Rimola A, Navasa M, Arroyo V. (1995) Experience with cefotaxime in the treatment of spontaneous bacterial peritonitis in cirrhosis. Diagn Microbiol Infect Dis. 22(1-2): 141-145. [PubMed.]
- Todd PA, Brogden RN. (1990) Cefotaxime. An update of its pharmacology and therapeutic use. Drugs. 40(4): 608-651. [PubMed.]
- Reeves JH, Russell GM, Cade JF, McDonald M. (1989) Comparison of ceftriaxone with cefotaxime in serious chest infections. Chest. 96(6): 1292-1297. [PubMed.]
- Carmine AA, Brogden RN, Heel RC, Speight TM, Avery GS. (1983) Cefotaxime. A review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs. 25(3): 223-289. [PubMed.]
- Brogden RN, Spencer CM. (1997) Cefotaxime. A reappraisal of its antibacterial activity and pharmacokinetic properties, and a review of its therapeutic efficacy when administered twice daily for the treatment of mild to moderate infections. Drugs. 53(3): 483-510. [PubMed.]
- LeFrock JL, McCloskey RV. (1987) Cefotaxime treatment of skin and skin structure infections: a multicenter study. Clin Ther. 82(4A): 242-246. [Ref.]
- Pérez-Ruvalcaba JA, Quintero-Pérez NP, Morales-Reyes JJ, Huitrón-Ramírez JA, Rodríguez-Chagollán JJ, et al. (1987) Double-blind comparison of ciprofloxacin with cefotaxime in the treatment of skin and skin structure infections. Am J Med. 82(4A): 242-246. [PubMed.]
- Grassi C, Colombo ML, Peona V. (1983) The use of cefotaxime in pneumology. Acta Med Port. 4(2): 87-90. [PubMed.]
- Cordero E, Bouza E, Ruiz I, Pachon J. (2001) Cefepime versus cefotaxime for empirical treatment of bacterial pneumonia in HIV-infected patients: an open, randomized trial. J Antimicrob Chemother. 48(4): 527-534. [PubMed.]
- Rimola A, Salmerón JM, Clemente G, Rodrigo L, Obrador A, et al. (1995) Two different dosages of cefotaxime in the treatment of spontaneous bacterial peritonitis in cirrhosis: results of a prospective, randomized, multicenter study. Hepatology. 21(3): 674-679. [PubMed.]
- Fourcade RO. (1990) Antibiotic prophylaxis with cefotaxime in endoscopic extraction of upper urinary tract stones: a randomized study. The Cefotaxime Cooperative Group. J Antimicrob Chemother. 26(Suppl A): 77-83. [PubMed.]
- Gentry LO, Ramirez-Ronda CH, Rodriguez-Noriega E, Thadepalli H, del Rosal PL, et al. (1989) Oral ciprofloxacin vs parenteral cefotaxime in the treatment of difficult skin and skin structure infections. A multicenter trial. Arch Intern Med. 149(11): 2579-2583. [PubMed.]
- Perkins RL. (1982) Clinical trials of cefotaxime for the treatment of bacterial infections of the lower respiratory tract. Rev Infect Dis. 4(Suppl 4): S421-S431. [PubMed.]
- Madsen PO. (1982) Treatment of urinary tract infections with cefotaxime: noncomparative and prospective comparative trials. Rev Infect Dis. 4(Suppl 4): S416-S420. [PubMed.]
- Smith CR, Ambinder R, Lipsky JJ, Petty BG, Israel E, et al. (1984) Cefotaxime compared with nafcillin plus tobramycin for serious bacterial infections. A randomized, double-blind trial. Ann Intern Med. 101(4): 469-477. [PubMed.]
- Nau R, Prange HW, Muth P, Mahr G, Menck S, et al. (1993) Passage of cefotaxime and ceftriaxone into cerebrospinal fluid of patients with uninflamed meninges. Antimicrob Agents Chemother. 37(7): 1518-1524. [Ref.]
- Turnier PL, Helali NEN, Guilhaumou R, Pilmis B, Revest M, et al. (2021) CSF concentration of cefotaxime in adult patients with pneumococcal meningitis: a multicentre retrospective study. J Antimicrob Chemother. 76(9): 2352-2355. [PubMed.]
- Goldwater PN. (2005) Cefotaxime and ceftriaxone cerebrospinal fluid levels during treatment of bacterial meningitis in children. Int J Antimicrob Agents. 26(5): 408-411. [PubMed.]
- Mullaney DT, John JF. (1983) Cefotaxime therapy. Evaluation of its effect on bacterial meningitis, CSF drug levels, and bactericidal activity. Arch Intern Med. 143 (9): 1705-1708. [Ref.]
- Viladrich PF, Cabellos C, Pallares R, Tubau F, Martínez-Lacasa J, et al. (1996) High doses of cefotaxime in treatment of adult meningitis due to Streptococcus pneumoniae with decreased susceptibilities to broad-spectrum cephalosporins. Antimicrob Agents Chemother. 40(1): 218-220. [PubMed.]
- Jacob RF, Wells TG, Steele RW, Yamauchi T. (1985) A prospective randomized comparison of cefotaxime vs ampicillin and chloramphenicol for bacterial meningitis in children. J Pediatr. 107(1): 129-133. [PubMed.]
- Scholz H, Hofmann T, Noack R, Edwards DJ, Stoeckel K. (1998) Prospective comparison of ceftriaxone and cefotaxime for the short-term treatment of bacterial meningitis in children. Chemotherapy. 44(2): 142-147. [PubMed.]
- Wells TG, Trang JM, Brown AL, Marmer BC, Jacobs RF. (1984) Cefotaxime therapy of bacterial meningitis in children. J Antimicrob Chemother. 14(Suppl B): 181-189. [PubMed.]
- Haffejee IE. (1988) Cefotaxime versus penicillin-chloramphenicol in purulent meningitis: a controlled single-blind clinical trial. Ann Trop Paediatr. 8(4): 225-229. [PubMed.]
- Nürnberg S, Claus H, Krone M, Vogel U, Lâm T-T. (2021) Cefotaxime resistance in invasive Haemophilus influenzae isolates in Germany 2016-19: prevalence, epidemiology and relevance of PBP3 substitutions. J Antimicrob Chemother. 76(4): 920-929. [PubMed.]
- Vinueza-Burgos C, Ortega-Paredes D, Narváez C, De Zutter L, Zurita J. (2019) Characterization of cefotaxime resistant Escherichia coli isolated from broiler farms in Ecuador. PLoS One. 14(4): e0207567. [Ref.]
- Mir RA, Weppelmann TA, Johnson JA, Archer D, J Morris G Jr 2, et al. (2016) Identification and Characterization of Cefotaxime Resistant Bacteria in Beef Cattle. PLoS One. 11(9): e0163279. [Ref.]
- Bonomo RA, Donskey CJ, Blumer JL, Hujer AM, Hoyenm CK, et al. (2003) Cefotaxime-resistant bacteria colonizing older people admitted to an acute care hospital. J Am Geriatr Soc. 51(4): 519-522. [PubMed.]
- Chuang Y-M, Tseng S-P, Teng L-J, Y-C, Hsueh P-R. (2006) Emergence of cefotaxime resistance in Citrobacter freundii causing necrotizing fasciitis and osteomyelitis. J Infect. 53(3): e161-e163. [PubMed.]
- Brown CE, Christmas JT, Bawdon RE. (1990) Placental transfer of cefotaxime and piperacillin in pregnancies remote from term complicated by Rh isoimmunisation. Am J Obstet Gynecol. 163(3): 938-943. [PubMed.]
- Kafetzis DA, Lazarides CV, Siafas CA, Georgakopoulos PA, Papadatos CJ. (1980) Transfer of cefotaxime in human milk and from mother to foetus. J Antimicrob Chemother. 6(Suppl A): 135-141. [PubMed.]
- Kafetzis DA, Siafas CA, Georgakopoulos PA, Papadatos CJ. (1981) Passage of cephalosporins and amoxicillin into the breast milk. Acta Paediatr Scand. 70(3): 285-288. [PubMed.]
- Takase Z, Fujiwara M, Kohmoto Y, Seto M, Shirafuji H. (1982) Study of cefotaxime in the perinatal period. Jpn J Antibiot. 35(7): 1893-1897. [PubMed.]
