>Corresponding Author : Gian Maria Pacifici
>Article Type : Mini Review Article
>Volume : 2 | Issue : 1
>Received Date : 26 Nov, 2022
>Accepted Date : 12 Dec, 2022
>Published Date : 18 Dec, 2022
>DOI : https://doi.org/10.54289/JCTRE2200103
>Citation : Pacifici GM. (2022) Clinical Pharmacology of Antiretroviral Agents in Infants and Children. J Clin Trials Res Ethics 2(1): doi https://doi.org/10.54289/JCTRE2200103
>Copyright : © 2022 Pacifici GM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Mini Review Article | Open Access
Associate Professor of Pharmacology via Sant’Andrea 32, 56127 Pisa, Italy
*Corresponding author: Gian Maria Pacifici, Associate Professor of Pharmacology via Sant’Andrea 32, 56127 Pisa, Italy
Abstract
The antiretroviral agents used in paediatric patients are: zidovudine, lamivudine, stavudine, and tenofovir disoproxil. The dosing of zidovudine, lamivudine, stavudine, and tenofovir disoproxil has been reviewed. The pharmacokinetics of zidovudine have been studied in children and the mean elimination half-life is 3.27 hours. Zidovudine treats pregnant women affected by HIV and prevents the transmission of HIV from the mother to the infant. The pharmacokinetics of lamivudine have been studied in infants and children and the elimination half-life ranges from 3.8 to 6.2 hours being longer in infants than in children. Lamivudine treats women infected by the hepatitis B virus and prevents the transmission of hepatitis B virus from the mother to the infant. The pharmacokinetics of stavudine have been studied in infants aged 1 and 6 weeks and the mean elimination half-life is 135 and 91.6 min being longer in younger than older infants. The pharmacokinetics of tenofovir disoproxil fumarate have been studied in infants and the median elimination half-life is 16.9 hours. Tenofovir disoproxil fumarate treats pregnant women infected by hepatitis B virus and prevents the transmission of hepatitis B virus from the mother to the infant. The aim of this study is to review the dosing of zidovudine, lamivudine, stavudine, and tenofovir disoproxil, the pharmacokinetics of zidovudine, lamivudine, stavudine, and tenofovir disoproxil fumarate, the transfer across the human placenta of zidovudine, lamivudine, and tenofovir, and the migration into the breast-milk of zidovudine, lamivudine, stavudine, and tenofovir disoproxil fumarate.
Keywords: Breast milk; Efficacy-Safety; Lamivudine; Pharmacokinetics; Placenta; Stavudine; Tenofovir-Disoproxil; Treatments; Zidovudine
Introduction
The antiretroviral agents used in paediatric patients are: zidovudine, lamivudine, stavudine, and tenofovir disoproxil.
Mechanism of action of antiretroviral agents
Intracellular zidovudine is phosphorylated to zidovudine 5’-thriphosphate zidovudine. Zidovudine-triphosphate terminates the elongation of proviral DNA because it is incorporated by reverse transcriptase into nascent DNA but lacks a 3’-hydroxyl group. The monophosphate competitively inhibits cellular thymidylate kinase, and this may reduce the amount of intracellular thymidine triphosphate formed. Zidovudine-triphosphate only weakly inhibits cellular DNA polymerase α but is a more potent inhibitor of mitochondrial polymerase γ. Because the conversion of zidovudine-monophosphate to diphosphate is inefficient, high concentrations of the monophosphate accumulate inside cells and may serve as a precursor depot for formation of triphosphate. As a consequence, there is little correlation between extracellular concentrations of parent drug and intracellular concentrations of triphosphate, and higher plasma concentrations of zidovudine do not increase intracellular triphosphate concentrations proportionately. Lamivudine enter cells by passive diffusion and is sequentially phosphorylated to lamivudine 5’-triphosphate, which is the active metabolite. Lamivudine has low affinity for human DNA polymerases, explaining its low toxicity to the host. High-level resistance to lamivudine occurs with single-amino-acid substitution, M184V orM181I. These mutations can reduce in-vitro sensitivity to lamivudine as much as 1000-fold. The MI84V mutation restores zidovudine susceptibility in zidovudine-resistant HIV harbouring the K65R mutation. This effect may contribute to the sustained virological benefits of zidovudine and lamivudine combination therapy. Intracellular stavudine is sequentially phosphorylated to form stavudine 5’-triphosphate. Like zidovudine, stavudine is most potent in activated cells, probably because thymidine kinase, which produces the monophosphate, is an S-phase-specific enzyme. Stavudine resistance is seen most frequently with mutations at reverse transcriptase codons 41, 44, 67, 70, 210, 215, and 219, which are mutations associated with zidovudine resistance. Resistance mutations for stavudine analogues have been reported following prolonged therapy. Tenofovir disoproxil is hydrolysed rapidly to tenofovir, which is phosphorylated by cellular kinases to its active metabolite, tenofovir diphosphate, which is actually a triphosphate. The parent drug is a monophosphate. The disposition of oral tenofovir alafenamide is similar, but it circulates largely as the uncleaved prodrug, which is taken up into cells and then converted to the parent nucleotide; as a consequence, circulating concentrations of tenofovir, which may contribute to renal toxicity, are much lower than produced by the disoproxil fumarate prodrug. Tenofovir diphosphate is a competitive of viral reverse transcriptases and is incorporated into HIV DNA to cause chain termination because it has an incomplete ribose ring. Although tenofovir diphosphate has broad-spectrum activity against viral DNA polymerase α, polymerase β, and polymerase γ, which is the basis for its selective toxicity [1].
Literature search
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “zidovudine children, “lamivudine children, “stavudine children”, and “tenofovir disoproxil Children”: In addition the book “The Pharmacological Basis of the Therapeutics” [1] has been consulted.
Results
Zidovudine molecular structure (molecular weight = 267.242 grams/mole)
Administration of zidovudine to children [2]
Oral administration of zidovudine to treat HIV infection in combination with other antiretroviral drugs
Administration to children
Children. Give: 180 mg/m2 twice-daily (maximum per dose = 300 mg).
Children with body-weight of 8 to 13 kg. Give: 100 mg twice-daily.
Children with body-weight of 14 to 20 kg. Give: 100 mg, to be taken in the morning and 200 mg to be taken in the evening.
Children with body-weight of 21 to 27 kg. Give: 200 mg twice-daily.
Children with body-weight of 28 to 29 kg. Give: 200 to 250 mg twice-daily.
Children with body-weight of 30 kg and above. Give: 200 to 250 mg twice-daily.
Oral administration of zidovudine to treat HIV infection in combination with other antiretroviral drugs
Administration to children
Children with body-weight of 4 to 8 kg. Give: 12 mg/kg twice-daily.
Children with body-weight of 9 to 29 kg. Give: 9 mg/kg twice-daily.
Intravenous infusion of zidovudine to treat HIV infection in combination with other antiretroviral drugs in children temporarily unable to take zidovudine by mouth
Administration to children
Children aged 3 months to 11 year. Give: 60 to 80 mg/m2 4 times-daily usually for not more than 2 weeks, the dose approximating to 9 to 12 mg/kg twice-daily by mouth.
Children aged 12 to 17 years. Give: 0.8 to 1 mg/kg 6 times-daily usually for not more than 2 weeks, the dose approximately to 1.2 to 1.5 mg/kg 6 times-daily by mouth.
Efficacy and safety of zidovudine in infants and children
Zidovudine was administered orally at a dose of 2 mg/kg 4 times-daily to infants aged less than 2 weeks and at a dose of 3 mg/kg 4 times-daily to infants older than 2 weeks. Infants were infected by HIV and zidovudine effectively and safe treats these infants [3]. Zidovudine was administered orally at a dose of 250 mg twice-daily to pregnant women infected by HIV, this treatment is well-tolerated, gives improvements in CD4, and zidovudine effectively and safe prevents the transmission of HIV from the mother to the infant [4]. Zidovudine was administered orally at a dose of 180 mg/m2 4 times-daily to children infected by HIV and this treatment effectively and safe treats children [5].
Fauchet et al. [6] studied the pharmacokinetics of zidovudine in 247 children, aged 6 months to 18 years, infected by HIV. Table 1 provides the dosages of zidovudine and table 2 summarizes the pharmacokinetic parameters of zidovudine.
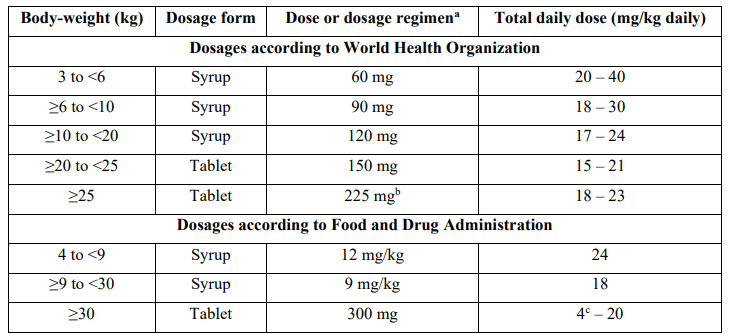
Table 1: Recommended dosages of zidovudine according to the Food and Drug Administration and according to the World Health Organization, by Fauchet et al. [6].
aAll dosages are given twice-daily.
bPatients received an oral dose of 300 mg every morning and 150 mg every evening.
cFor an adult weighing 150 kg.
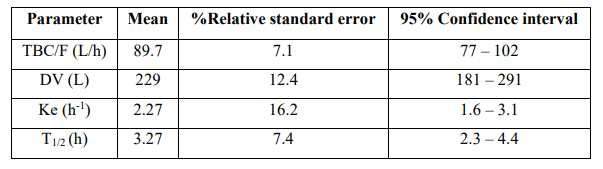
Table 2: Pharmacokinetic parameters of zidovudine which have been obtained in 247 children infected by HIV aged 6 months to 18 years and weighing 6 to 85 kg. Values are the mean, %relative standard error, and 95% confidence interval, by Fauchet et al. [6].
TBC = total body clearance. F = bioavailability. DV = distribution volume. Ke = elimination-rate constant. T1/2 = elimination half-life.
This table shows that the distribution volume of zidovudine is larger than the water volume, and zidovudine is slowly eliminated as the mean elimination-rate constant and the mean elimination half-life are 2.27 h-1 and 3.27 h, respectively.
Treatment of pregnant women affected by HIV with zidovudine combined to other antiretroviral drugs and prevention of transmission of HIV from the mother to the infant
Zidovudine, administered orally at a dose of 250 mg twice-daily to HIV infected women, reduces the risk of maternal to infant transmission of HIV by approximately two thirds [7]. Lamivudine and zidovudine effectively prevents the HIV transmission form the mother to the infant [8]. Mothers received a 200 mg single oral dose of nevirapine intrapartum and infants received a single oral dose of 2 mg/kg of nevirapine plus 4 mg/kg twice-daily zidovudine for a week. This treatment prevents the HIV transmission from the mother to the infant [9]. Zidovudine, monotherapy or in combination with other antiretroviral agents, remains a first-choice therapy for the prevention of HIV transmission from the mother to the infant [10].
Transfer of zidovudine across the human placenta
Zidovudine was administered orally at a dose of 200 mg twice-daily to 26 pregnant women at labour. Zidovudine crosses the first trimester human placenta readily and zidovudine concentration in cord serum is similar to the maternal serum concentration [11]. The transfer of zidovudine across human placenta was studied using an in-vitro perfusion system with independent maternal and foetal circuits and zidovudine concentration rapidly equilibrates between the maternal and foetal compartment [12]. These results indicate that zidovudine freely crosses the human placenta.
Migration of zidovudine into the breast-milk
Eighteen lactating women received zidovudine orally at a dose of 300 mg twice-daily and the median zidovudine concentration in milk is 207 µg/ml [13]. Thirty-eight lactating women received zidovudine orally at a dose of 300 mg twice-daily and the median concentration of zidovudine in the milk is 33 µg/ml (range, 5 to 117) at a median time after the dose of 4.5 hours (range, 3.5 to 6) [14]. Nine HIV-positive lactating women received 3 oral doses of 300 mg of zidovudine and the average milk concentration of zidovudine is 101 µg/ml (range, 20 to 448) [15]. These results indicate that zidovudine migrates into the breast-milk in significant amounts.
Lamivudine molecular structure (molecular weight = 229.26 grams/mole)
Administration of lamivudine to children [16]
Oral administration of lamivudine to treat HIV infection in combination with other antiretroviral drugs
Administration to children
Children aged 1 to 2 months. Give: 4 mg/kg twice-daily.
Children aged 3 months to 11 years with body-weight up to 14 kg. Give: 4 mg/kg twice-daily (maximum per dose = 300 mg), alternatively give 8 mg/kg once-daily (maximum per dose = 300 mg).
Children aged 3 months to 11 years with body-weight of 15 to 20 kg. Give: 4 mg/kg twice-daily (maximum per dose = 150 mg), alternatively give 8 mg/kg once-daily (maximum per dose = 300 mg), alternatively give 75 mg twice-daily, alternatively give 150 mg once-daily.
Children aged 3 months to 11 years with body-weight of 21 to 29 kg. Give: 4 mg/kg twice-daily (maximum per dose = 150 mg), alternatively give 8 mg/kg once-daily (maximum per dose = 300 mg), alternatively give 75 mg daily, the dose should be taken in the evening, alternatively give 225 mg once-daily.
Children aged 3 months to 11 years with body-weight of 30 kg and above. Give: 4 mg/kg twice-daily (maximum per dose = 150 mg), alternatively give 8 mg/kg once-daily (maximum per dose = 300 mg), alternatively give 150 mg twice-daily, alternatively give 300 mg once-daily.
Children aged 12 to 17 years. Give: 150 mg twice-daily, alternatively give 300 mg once-daily.
Oral administration of lamivudine to treat HIV infection in combination with other antiretroviral drugs
Administration to children
Children aged 1 to 2 months. Give: 4 mg/kg twice-daily.
Children aged 3 months to 11 years with a body-weight up to 14 kg. Give: 4 mg/kg twice-daily, alternatively give 8 mg/kg once-daily.
Children aged 3 months to 11 years with a body-weight of 15 to 20 kg. Give: 4 mg/kg twice-daily (maximum per dose = 150 mg), alternatively give 8 mg/kg once-daily (maximum per dose = 300 mg), alternatively give 75 mg twice-daily, alternatively give 150 mg once-daily.
Children aged 3 months to 11 years with a body-weight of 21 to 29 kg. Give: 4 mg/kg twice-daily (maximum per dose = 150 mg), alternatively give 8 mg/kg once-daily (maximum per dose = 300 mg), alternatively give 75 mg daily, the dose should be taken in the morning and 150 mg should be taken in the evening, alternatively give 225 mg once-daily.
Children aged 3 months to 11 years with a body-weight of 30 kg and above. Give: 4 mg/kg twice-daily (maximum per dose = 150 mg), alternatively give 8 mg/kg once-daily (maximum per dose = 300), alternatively give 150 mg twice-daily, alternatively give 300 mg once-daily.
Children aged 12 to 17 years. Give: 150 mg twice-daily, alternatively give 300 mg once-daily.
Oral administration of lamivudine to treat hepatitis B infection either with compensated liver disease (with evidence of viral replication and histology of active liver inflammation or fibrosis) when first-line treatment cannot be used, or (in combination with another antiretroviral drug without cross-resistance to lamivudine) with decompensated liver disease
Administration to children
Children aged 2 to 11 years. Give: 3 mg/kg once-daily (maximum per dose = 100 mg), children receiving lamivudine for concomitant HIV infection should continue to receive lamivudine in a dose appropriate for HIV infection.
Children aged 12 to 17 years. Give: 100 mg once-daily, children receiving lamivudine for concomitant HIV infection should continue to receive lamivudine in a dose appropriate for HIV infection.
Efficacy and safety of lamivudine in infants and children
Lamivudine, administered orally at a dose of 150 mg twice-daily to pregnant women at delivery, effectively and safe prevents the transmission of HIV type 1 from the mother to the infant [17]. Lamivudine, administered orally at a dose of 4 mg/kg twice-daily, effectively and safe treats infants infected by hepatitis B virus [18]. Lamivudine was administered orally at a dose of 3 mg/kg daily to children infected by the hepatitis B virus and lamivudine effectively and safety treats children infected by chronic hepatitis B virus [19]. Lamivudine, administered orally at a dose of 4 mg/kg twice-daily, effectively and safe treats children infected by HIV [20].
Pharmacokinetics of lamivudine in infants
Tremoulet et al. [21]. studied the pharmacokinetics of lamivudine in 99 term infants with HIV disease and lamivudine was administered orally at a dose of 2 mg/kg twice-daily for the first 4 to 6 weeks of life and at a dose of 4 mg/kg twice-daily thereafter. Table 3 summarizes the pharmacokinetic parameters of lamivudine.
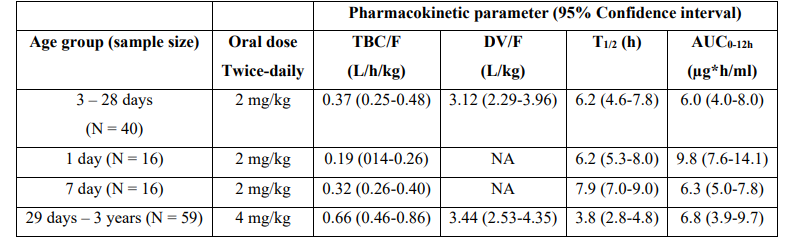
Table 3: Pharmacokinetic parameters of lamivudine in HIV-exposed and infected infants and children. Values are the median and (interquartile range), by Tremoulet et al. [21].
TBC = total body clearance. F = bioavailability. DV = distribution volume. T1/2 = elimination half-life. AUC = area under the concentration-time curve. NA = not applicable.
This table shows that the median total body clearance is higher in the 4th age group than in the other age groups, the distribution volume is larger than the water volume, the median elimination half-life is shorter in the 4th age group than in the other age groups, and the median area under the concentration-time curve is similar in the 4 age groups. In addition, there is a remarkable interindividual variability in the pharmacokinetic parameters and this variability is accounted by the wide variation in infant age and disease.
Treatment of pregnant women and children infected by hepatitis B virus with lamivudine and prevention of transmission of hepatitis B virus from the mother to the infant
Lamivudine, administered orally at a dose of 4 mg/kg twice-daily, effectively treats chronic hepatitis B virus in pregnant women and prevents the transmission of hepatitis B virus from the mother to infant [22]. Lamivudine, administered orally at a dose of 300 mg once-daily, prevents the transmission of hepatitis B virus from the mother to the infant [23]. Lamivudine, administered orally at a dose of 4 mg/kg twice-daily to pregnant women, interrupts the transmission of hepatitis B virus from the mother to the infant [24]. Lamivudine, administered orally at a dose of 4 mg/kg twice-daily to 45 children infected by chronic hepatitis B virus, effectively treats children infected by chronic hepatitis B virus [25]. Ninety children received lamivudine at a dose of 1 to 20 mg/kg daily, the children were infected by HIV, and lamivudine is well-tolerated and exhibits virological activity in these children [26].
Transfer of lamivudine across the human placenta
Lamivudine was administered orally at a dose of 150 mg to 57 pregnant women at delivery. Lamivudine median maternal and fetal plasma concentrations are 302 and 240 ng/ml, respectively. Individual maternal and fetal concentrations are strongly correlated (r2 = 0.36; P-value < 10-4), the median ratio is about 1, and lamivudine crosses the placenta by simple diffusion [27]. These results indicate that lamivudine is freely transferred from the mother to the foetus.
Migration of lamivudine into the breast-milk
Lamivudine was administered orally at a dose of 300 mg twice-daily to 10 lactating women. The average milk concentration of lamivudine is 1.2 µg/ml (range, < 5 to 6.1) [28]. Lamivudine was administered for 3 days postpartum and the average milk concentration of lamivudine in milk is < 20 µg/ml [29]. Fifty-eight lactating women received lamivudine orally at a dose of 150 mg twice-daily and the median lamivudine concentration in milk is 1.2 µg/ml [30]. These results indicate that lamivudine poorly migrates into the breast-milk.
Stavudine molecular structure (molecular weight = 224.213 grams/mole)
Administration of stavudine to children [31]
Oral administration of stavudine to treat HIV infection in combination with other antiretroviral drugs when no suitable alternative is available and prescribed for shortest period possible
Administration to children
Children with body-weight up to 30 kg. Give: 1 mg/kg twice-daily, the dose should be taken preferable 1 hour before food.
Children with body-weight of 60 kg and above. Give: 40 mg twice-daily, the dose should be taken preferable 1 hour before food.
Pharmacokinetics of stavudine in infants
Wade et al. [32] studied the pharmacokinetics of stavudine in 10 infants infected by HIV aged 1 week and in 10 infants infected by HIV aged 6 weeks. Stavudine was administered orally at a dose of 1 mg/kg once-daily. Infants had a median body-weight of 3,167 grams (range, 2,200 to 3,915) and had a median gestational age of 38 (range, 35 to 40).
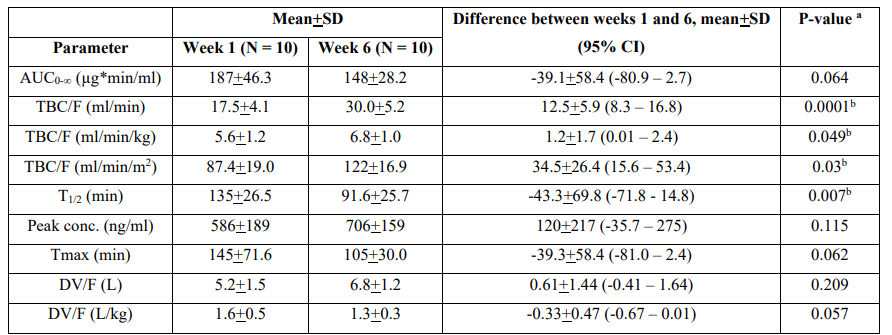
Table 4:Pharmacokinetic parameters of stavudine which have been obtained in HIV infected infants who received a single oral dose of 1 mg/kg stavudine and infants were aged 1 and 6 weeks. Values are the mean+SD and the (95% confidence interval) by Wade et al. [32].
AUC = area under the concentration-tine curve. T1/2 = elimination half-life. Tmax = time to reach the peak concertation. DV = distribution volume. F = bioavailability. TBC = total body clearance. a Student t test for unpaired data. b P-value < 0.05.
This table shows that stavudine is rapidly eliminated and the distribution volume is similar to the water volume. The total body clearance of stavudine is higher in older than in younger infants consequently the elimination half-life is shorter in older than younger infants. In addition, there is a remarkable interindividual variability in the pharmacokinetic parameters and this variability is accounted by a wide variation of infant age and disease.
Migration of stavudine into the breast-milk
Stavudine was administered orally at a dose of 30 mg once-daily to 52 lactating women and the median concentration of stavudine in milk is 151 µg/ml [33]. These results indicate that stavudine migrates into the breast-milk in significant amounts.
Tenofovir disoproxil molecular structure (molecular weight = 287.213 grams/mole)
Tenofovir disoproxil fumarate molecular structure (molecular weight = 635.5159 grams/mole)
Administration of tenofovir disoproxil to children [34]
Oral administration of tenofovir disoproxil to treat HIV infection in combination with other antiretroviral drugs when the first-line nucleoside reverse transcriptase inhibitors cannot be used because of resistance or contra-indications
Administration to children
Children aged 2 to 17 years. Give: 6.5 mg/kg once-daily (maximum per dose = 245 mg).
Children aged 6 to 17 years with body-weight of 17 to 21 kg. Give: 123 mg once-daily.
Children aged 6 to 17 years with body-weight of 22 to 27 kg. Give: 163 mg once-daily.
Children aged 6 to 17 years with body-weight of 28 to 34 kg. Give: 204 mg once-daily.
Children aged 6 to 17 years with body-weight of 35 kg and above. Give: 245 mg once-daily.
Oral administration of tenofovir disoproxil to treat chronic hepatitis B infection with compensated liver disease (with evidence of viral replication, and histology of active liver inflammation or fibrosis)
Administration to children
Children aged 12 to 17 years with body-weight of 35 kg and above. Give: 245 mg once-daily.
Pharmacokinetics of tenofovir disoproxil fumarate in infants
Flynn et al. [35] studied the pharmacokinetics of tenofovir disoproxil fumarate in 25 infants infected by HIV-1. A single oral dose of 600 mg of tenofovir disoproxil fumarate was administered to 10 infants and a single oral dose of tenofovir disoproxil fumarate of 900 mg was administered to 15 infants.
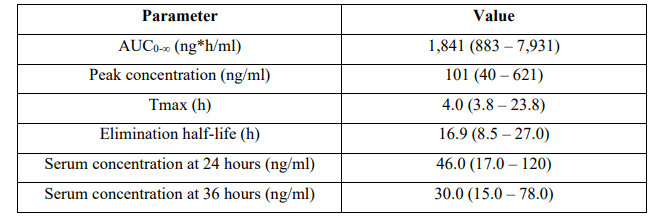
Table 5: Pharmacokinetic parameters of tenofovir disoproxil fumarate which have been obtained in all infants who were infected by HIV-1. Values are the median and (range) by Flynn et al. [35].
AUC = area under the concentration-time curve. Tmax = time to reach the peak concentration.
This table shows that tenofovir disoproxil fumarate is slowly absorbed as the median time to react the peak concentration is 4.0 hours and tenofovir disoproxil fumarate is slowly eliminated as the median elimination half-life is 16.9 hours. In addition, there is a remarkable interindividual variability in the pharmacokinetic parameters and this variability is accounted by a wide variation in infant age and disease.
Treatment of pregnant women infected by the hepatitis B virus with tenofovir disoproxil fumarate and prevention of transmission of hepatitis B virus from the mother to the infant
Tenofovir disoproxil fumarate significantly reduces the rate of transmission of hepatitis B virus from the mother to the infant [36]. In pregnant women who have high hepatitis B virus DNA titers, tenofovir disoproxil fumarate reduces the transmission of hepatitis B virus from mother to the infant [37]. Treatment with tenofovir disoproxil fumarate initiated at 24 weeks of gestation effectively prevents the transmission of hepatitis B virus from the mother to the infant [38]. Tenofovir disoproxil fumarate effectively and safe prevents the transmission of hepatitis B virus from the mother to the infant [39]. Tenofovir disoproxil fumarate therapy during the second or third trimester of pregnancy reduces the perinatal transmission of hepatitis B virus from the mother to the infant [40].
Transfer of tenofovir across the human placenta
Tenofovir 1% vaginal gel was applied to the vagina preoperatively to 16 women undergoing Caesar section and the peak concentration of tenofovir is 4.3 and 1.9 ng/ml in the maternal serum and in the cord vein serum, respectively, thus tenofovir is poorly transferred across the human placenta [41].
Migration of tenofovir disoproxil fumarate into the breast-milk
Five lactating women received tenofovir disoproxil fumarate orally at a dose of 300 mg once-daily and the median peak and trough concentration of tenofovir disoproxil fumarate in the milk is 14.1 and 6.8 µg/ml, respectively [42]. Twenty-five lactating women received a single oral dose of 600 or 900 mg of tenofovir disoproxil fumarate and tenofovir disoproxil fumarate concentration in the milk ranges from 6.3 to 17.8 µg/ml [43]. Fifty lactating women received a single oral dose of 300 mg of tenofovir disoproxil fumarate and the median peak and trough concentrations of tenofovir disoproxil fumarate are 3.2 and 3.3 µg/ml, respectively [44]. Forty-eight lactating women received a single oral dose of tenofovir disoproxil fumarate of 300 mg and the median concentration of tenofovir disoproxil fumarate is 5.98 µg/ml at a median time of 4 hours [45]. These results indicate that tenofovir disoproxil fumarate poorly migrates into the breast-milk.
Discussion
The antiretroviral agents used in paediatric patients are: zidovudine, lamivudine, stavudine, and tenofovir disoproxil. Zidovudine, lamivudine, and tenofovir disoproxil have been extensively studied in children whereas little information of stavudine is available which consists in pharmacokinetics and in migration into the breast-milk. The dosing of zidovudine, lamivudine, stavudine, and tenofovir disoproxil has been reviewed. Zidovudine has been found efficacy and safe in infants and children [3-5], the pharmacokinetics of zidovudine have been studied in children and the elimination half-life of zidovudine is 3.27 hours [6]. Zidovudine treats pregnant women affected by HIV and prevents the transmission of HIV from the mother to the infant [7-10], is freely transferred across the human placenta [11, 12], and migrates into the breast-milk in significant amounts [13-15]. Lamivudine has been found efficacy and safe in infants and children [17-20], the pharmacokinetics of lamivudine have been studied in infants and children and the elimination half-life of lamivudine ranges from 3.8 to 7.9 hours being longer in infants than in children [21]. Lamivudine treats pregnant women affected by the hepatitis B virus, prevents the transmission of hepatitis B virus from the mother to the infant [22-26], is freely transferred across the human placenta [27], and poorly migrates into the breast-milk [28-30]. The pharmacokinetics of stavudine have been studied in infants aged 1 week and in infants aged 6 weeks and the elimination half-life of stavudine is 135 and 92.6 min being longer in the younger than older infants [32] and stavudine migrates into the breast-milk in significant amounts [33]. The pharmacokinetics of tenofovir disoproxil fumarate have been studied in infants and the elimination half-life of tenofovir disoproxil fumarate is 16.9 hours [35]. Tenofovir disoproxil fumarate treats women infected by hepatitis B virus and prevents the transmission of hepatitis B virus from the mother to the infant [36-40]. Tenofovir is poorly transferred across the human placenta [41] and tenofovir disoproxil fumarate poorly migrates into the breast-milk [42-45].
In conclusion, the antiretroviral used in paediatric patients are: zidovudine, lamivudine, stavudine, and tenofovir disoproxil. Zidovudine and lamivudine have been found efficacy and safe in infants and children. The pharmacokinetics of zidovudine have been studied in children and the pharmacokinetics of lamivudine, stavudine, and tenofovir disoproxil fumarate have been studied in infants. Zidovudine and lamivudine are freely transferred across the human placenta whereas and tenofovir is poorly transferred across the human placenta. Zidovudine and stavudine migrate into the breast-milk in significant amounts whereas lamivudine and tenofovir disoproxil fumarate poorly migrates into the breast-milk. The aim of this study is to review the clinical pharmacology of zidovudine, lamivudine, stavudine, and tenofovir disoproxil in infants and children.
Conflict of interests
The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria.
This article is a review and drugs have not been administered to men or animals.
Acknowledgments
The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.
References
- Flexner CW. (2018) “Antiretroviral Agents and Treatment of HIV infection”. In The Goodman & Gilman’s. The Pharmacological Basis of the Therapeutics, Brunton Hilal-dandan LL, Knollmann BC, editors, USA. 1137-1157. [Ref.]
- The British national formulary for children. (2019-2020) “Zidovudine”. Macmillan, 78th Edition, Hampshire International Business Park, Hampshire, Lime Three Way, Basingstoke, Hampshire, UK. 438. [Ref.]
- Boucher FD, Modlin JF, Weller S, Ruff A, Mirochnick M, et al. (1993) Phase I evaluation of zidovudine administered to infants exposed at birth to the human immunodeficiency virus. J Pediatr. 122(1): 137-144. [PubMed.]
- Lambert JS, Nogueira SA, Abreu T, Machado ES, Costa TP, et al. (2003) A pilot study to evaluate the safety and feasibility of the administration of AZT/3TC fixed dose combination to HIV infected pregnant women and their infants in Rio de Janeiro, Brazil. Sex Transm Infect. 79(6): 448-452. [PubMed.]
- Brady MT, McGrath N, Brouwers P, Gelber R, Fowler MG, et al. (1996) Randomized study of the tolerance and efficacy of high- versus low-dose zidovudine in human immunodeficiency virus-infected children with mild to moderate symptoms (AIDS Clinical Trials Group 128). Pediatric AIDS Clinical Trials Group. J Infect Dis. 173(5): 1097-1106. [PubMed.]
- Fauchet F, Treluyer J-M, Frange P, Urien S, Foissac F, et al. (2013) Population pharmacokinetics study of recommended zidovudine doses in HIV-1-infected children. Antimicrob Agents Chemother. 57(10): 4801-4808. [Ref.]
- Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, et al. (1994) Reduction of Maternal-Infant Transmission of Human Immunodeficiency Virus Type 1 with Zidovudine Treatment. N Engl J Med. 331(11): 1173-1180. [Ref.]
- Mandelbrot L, Landreau-Mascaro A, Rekacewicz C, Berrebi A, Bénifla JL, et al. (2001) Lamivudine-zidovudine combination for prevention of maternal-infant transmission of HIV-1. JAMA. 285(16): 2083-2093. [PubMed.]
- Taha TE, Kumwenda NI, Hoover DR, Fiscus SA, Kafulafula G, et al. (2004) Nevirapine and zidovudine at birth to reduce perinatal transmission of HIV in an African setting: a randomized controlled trial. JAMA. 292(2): 202-209. [PubMed.]
- Bhana N, Ormrod D, Perry CM, Figgitt DP. (2002) Zidovudine: a review of its use in the management of vertically acquired pediatric HIV infection. Paediatr Drugs. 4(8): 515-553. [PubMed.]
- Siu S-S, Hok-Keung J, Man-Wah Y, Lau, Phil PYM, et al. (2005) Placental Transfer of Zidovudine in First Trimester of Pregnancy. Obstet Gynecol. 106(4): 720-727. [Ref.]
- Liebes L, Mendoza S, Wilson D, Dancis J. (1990) Transfer of Zidovudine (AZT) by Human Placenta. J Infect Dis. 161(2): 203-207. [PubMed.]
- Shapiro RL, Holland DT, Capparelli E, Lockman S, Thior I, et al. (2005) Antiretroviral concentrations in breast-feeding infants of women in Botswana receiving antiretroviral treatment. J Infect Dis. 192(5): 720-727. [PubMed.]
- Palombi L, Pirillo MF, Andreotti M, Liotta G, Erba F, Sagno J-B, et al. (2012) Antiretroviral prophylaxis for breastfeeding transmission in Malawi: drug concentrations, virological efficacy and safety. Antivir Ther. 17(8): 1511-1519. [PubMed.]
- Ramírez-Ramírez A, Sánchez-Serrano E, Loaiza-Flores G, Plazola-Camacho N, Rodríguez-Delgado RG, et al. (2018) Simultaneous quantification of four antiretroviral drugs in breast milk samples from HIV-positive women by an ultra-high performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) method. PLoS One. 13(1): e0191236. [PubMed.]
- The British national formulary for children. (2019-2020) “Lamivudine”. Macmillan, 78th Edition, Hampshire International Business Park, Hampshire, Lime Three Way, Basingstoke, Hampshire, UK. 436. [Ref.]
- Chaisilwattana P, Chokephaibulkit K, Chalermchockcharoenkit A, Vanprapar N, Sirimai K, et al. (2002) Short-course therapy with zidovudine plus lamivudine for prevention of mother-to-child transmission of human immunodeficiency virus type 1 in Thailand. Clin Infect Dis. 35(11): 1405-1413. [PubMed.]
- Zhang Y-T, Liu J, Pan X-B, Gao Y-D, Hu Y-F, et al. (2021) Successful treatment of infantile hepatitis B with lamivudine: A case report. World J Clin Cases. 9(14): 3442-3448. [PubMed.]
- Luo A, Jiang X, Ren H. (2019) Lamivudine therapy for chronic hepatitis B in children: a meta-analysis. Virol J. 16(1): 88. [Ref.]
- Perry CM, Faulds D. Lamivudine. (1997) A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in the management of HIV infection. Drugs. 53(4): 657-6580. [PubMed.]
- Tremoulet AH, Capparelli EV, Patel P, Acosta EP, Luzuriaga K, et al. (2007) Population Pharmacokinetics of Lamivudine in Human Immunodeficiency Virus- Exposed and –Infected Infants. Antimicrob Agents Chemother. 51(12): 4297-4302. [PubMed.]
- Yi W, Liu M, Cai H-D. (2012) Safety of lamivudine treatment for chronic hepatitis B in early pregnancy. World J Gastroenterol. 18(45): 6645-6650. [Ref.]
- van Zonneveld M, van Nunen AB, Niesters HGM, de Man RA, Schalm SW, et al. (2003) Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat. 10(4): 294-297. [PubMed.]
- Han L, Zhang H-W, Xie J-X, Zhang Q, Wang H-Y, et al. (2011) A meta-analysis of lamivudine for interruption of mother-to-child transmission of hepatitis B virus. World J Gastroenterol. 17(38): 4321-4333. [Ref.]
- Akman SA, Okcu SC, Halicioğlu O, Sutcuoglu S, Anil M, et al. (2007) Therapeutic efficacy of sequential and simultaneous treatments with interferon-alpha and lamivudine in children with chronic hepatitis B. Pediatr Int. 49(6): 848-852. [PubMed.]
- Lewis LL, Venzon D, Church J, Farley M, Wheeler S, et al. (1996) Lamivudine in children with human immunodeficiency virus infection: a phase I/II study. The National Cancer Institute Pediatric Branch-Human Immunodeficiency Virus Working Group. J Infect Dis. 174(1): 16-25. [PubMed.]
- Mandelbrot L, Peytavin G, Firtion G, Farinotti R. (2001) Maternal-fetal transfer and amniotic fluid accumulation of lamivudine in human immunodeficiency virus-infected pregnant women. Am J Obstet Gynecol. 184(2): 153-158. [PubMed.]
- Moodley J, Moodley D, Pillay K, Coovadia H, Saba J, et al. (1998) Pharmacokinetics and antiretroviral activity of lamivudine alone or when coadministered with zidovudine in human immunodeficiency virus type 1-infected pregnant women and their offspring. J Infect Dis. 178(5): 1327-1333. [PubMed.]
- Giuliano M, Guidotti G, Andreotti M, Pirillo MF, Villani P, et al. (2007) Triple antiretroviral prophylaxis administered during pregnancy and after delivery significantly reduces breast milk viral load: a study within the Drug Resource Enhancement Against AIDS and Malnutrition Program. Journal of J Acquir Immune Defic Syndr. 44(3): 286-291. [PubMed.]
- Mirochnick M, Thomas T, Capparelli E, Zeh C, Holland D, et al. Thigpen MC. (2009) Antiretroviral concentrations in breast-feeding infants of mothers receiving highly active antiretroviral therapy. Antimicrob Agents Chemother. 53(3): 1170-1176. [PubMed.]
- The British national formulary for children. (2019-2020) “Stavudine”. Macmillan, 78th Edition, Hampshire International Business Park, Hampshire, Lime Three Way, Basingstoke, Hampshire, UK. 437. [Ref.]
- Wade NA, Unadkat JD, Huang S, Shapiro DE, Mathias A, et al. (2004) Pharmacokinetics and safety of stavudine in HIV-infected pregnant women and their infants: Pediatric AIDS Clinical Trials Group protocol 332. J Infect Dis. 190(12): 2167-2174. [PubMed.]
- Fogel JM, Taha TE, Sun J, Hoover DR, Parsons TL, et al. (2012) Stavudine concentrations in women receiving postpartum antiretroviral treatment and their breastfeeding infants. J Acquir Immune Defic Syndr. 60(5): 462-465. [PubMed.]
- The British national formulary for children. (2019-2020) “Tenofovir disoproxil”. Macmillan, 78th Edition, Hampshire International Business Park, Hampshire, Lime Three Way, Basingstoke, Hampshire, UK. 437. [Ref.]
- Flynn PM, Mirochnick M, Shapiro DE, Bardeguez A, Rodman J, et al. (2011) Pharmacokinetics and safety of single-dose tenofovir disoproxil fumarate and emtricitabine in HIV-1-infected pregnant women and their infants. Antimicrob Agents Chemother. 55(12): 5914-5922. [PubMed.]
- Li W, Jia L, Zhao X, Wu X, Tang H. (2018) Efficacy and safety of tenofovir in preventing mother-to-infant transmission of hepatitis B virus: a meta-analysis based on 6 studies from China and 3 studies from other countries. BMC Gastroenterol. 161(2): 203-207. [Ref.]
- Lee Y-S, Lee HS, Kim JH, Chang SW, Hyun MH, et al. (2021) Role of tenofovir disoproxil fumarate in prevention of perinatal transmission of hepatitis B virus from mother to child: a systematic review and meta-analysis. Korean J Intern Med. 36(1): 76-85. [PubMed.]
- Lin Y, Liu Y, Ding G, Touqui L, Wang W, et al. (2018) Efficacy of tenofovir in preventing perinatal transmission of HBV infection in pregnant women with high viral loads. Sci Rep. 8(1): 15514. [Ref.]
- Chen J-Z, Liao Z-W, Huang F-L, Su R-K, Wang W-B, et al. (2017) Efficacy and safety of tenofovir disoproxil fumarate in preventing vertical transmission of hepatitis B in pregnancies with high viral load. Sci Rep. 7(1): 4132. [PubMed.]
- Celen MK, Mert D, Ay M, Dal T, Kaya S, et al. (2013) Efficacy and safety of tenofovir disoproxil fumarate in pregnancy for the prevention of vertical transmission of HBV infection. World J Gastroenterol. 19(48): 9377-9382. [Ref.]
- Beigi R, Noguchi L, Parsons T, Macio I, Ayudhya RPKN, et al. (2011) Pharmacokinetics and placental transfer of single dose tenofovir 1% vaginal gel in term pregnancy. J Infect Dis. 204(10): 1527-1531. [PubMed.]
- Benaboud S, Pruvost A, Coffie PA, Ekouévi DK, Urien S, et al. (2011) Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Cote d'Ivoire, in the ANRS 12109 TEmAA Study, Step 2. Antimicrob Agents Chemother. 55(3): 1315-1317. [PubMed.]
- Mirochnick M, Taha T, Kreitchmann R, Nielsen-Saines K, Kumwenda N, et al. (2014) Pharmacokinetics and safety of tenofovir in HIV-infected women during labor and their infants during the first week of life. J Acquir Immune Defic Syndr. 65(1): 33-41. [Ref.]
- Mugwanya KK, Hendrix CW, Mugo NR, Marzinke M, Katabira ET, et al. (2016) Pre-exposure Prophylaxis Use by Breastfeeding HIV-Uninfected Women: A Prospective Short-Term Study of Antiretroviral Excretion in Breast Milk and Infant Absorption. PLoS Med. 13(9): e1002132. [Ref.]
- Waitt C, Olagunju A, Nakalema S, Kyohaire I, Owen A, et al. (2018) Plasma and breast milk pharmacokinetics of emtricitabine, tenofovir and lamivudine using dried blood and breast milk spots in nursing African mother-infant pairs. J Antimicrob Chemother. 73(4): 1013-1019. [PubMed.]
