>Corresponding Author : Alan R George
>Article Type : Research Article
>Volume : 4 | Issue : 1
>Received Date : 23 Dec, 2023
>Accepted Date : 05 Jan, 2024
>Published Date : 09 Jan, 2024
>DOI : https://doi.org/10.54289/JDOE2400101
>Citation : George AR, Bruin DB, Retrum JK, Bane WE, Pfaff AS, et al. (2024) Blood Collection for Autologous Blood-Derived Product Preparation: Technique and Application. J Dent Oral Epidemiol 4(1): doi https://doi.org/10.54289/JDOE2400101
>Copyright : © 2024 Alan R George, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Research Article | Open Access
1Department of Periodontics, Army Postgraduate Dental School, Postgraduate Dental College, Uniformed Services University, Fort Eisenhower, GA
2Department of Periodontics, United States Army Dental Activity, Fort Leonard Wood, MO
3Department of Periodontics, United States Army Dental Activity, Fort Riley, KS
4Department of Periodontics, United States Army Dental Activity, Fort Wainwright, AK
5Department of Oral Pathology, Army Postgraduate Dental School, Tripler Army Medical Center, HI
*Corresponding author: Alan R George, Department of Periodontics, Army Postgraduate Dental School, Postgraduate Dental College, Uniformed Services University, Fort Eisenhower, GA
Abstract
Background: In periodontics, interest in use of autologous blood-derived products (ABPs) has increased due to demonstrated safety, enhanced initial healing, and for some applications, superior clinical outcomes. Periodontists commonly place peripheral intravenous catheters for moderate sedation, thus encountering opportunities to utilize ABPs routinely. The purpose of this report is to describe a safe blood collection technique for ABP preparation and define typical blood volume requirements for periodontal procedures.
Methods: Five cases requiring various amounts of liquid and membrane-formed platelet-rich fibrin are presented. Case 1 involves treatment of an infrabony periodontal defect. Case 2 illustrates alveolar ridge preservation at a maxillary right second premolar site. Case 3 demonstrates repair of a defect related to nasopalatine duct cyst removal. Cases 4 and 5 illustrate the use of PRF in sinus elevation and root coverage, respectively.
Results: Use of PRF in the presented cases added minimal procedural time and expense. Blood samples varied from 20 to 60 ml in volume. There were no complications related to blood collection or use of PRF. Each patient reported minimal discomfort limited to the first few postoperative days. Favorable early healing was observed in each case.
Conclusions: The blood collection method described in this report, which is consistent with published standards of practice, necessitates few additional steps and supplies for practitioners already placing peripheral intravenous catheters. Blood volumes necessary for typical procedures in periodontics are safe and well below maximum attainable samples.
Key words: Clinical Protocols; Biological Products; Blood; Blood Platelets; Platelet-Rich Fibrin; Treatment Outcome
Abbreviations: ABP: Autologous Blood-Derived Products, DFDBA: Demineralized Freeze-Dried Bone Allografts, PRP: Platelet-Rich Plasma, PRF: Platelet-Rich Fibrin, ARP/ARR: Alveolar Ridge Preservation/Reconstruction, IV: Intravenous,APDS: Army Postgraduate Dental School, FDBA: Freeze-Dried Bone Allograft, NDC: Nasopalatine Duct Cyst, SCTG: Subepithelial Connective Tissue Graft, TBV: Total Blood Volume, ED: Emergency Department, ANTT: Aseptic Non-Touch Technique
Introduction
It has been more than a half-century since Marshall Urist demonstrated the capacity of demineralized bone and dentin to induce bone formation when implanted into animal skin and muscle [1,2]. Dr. Gerald Bowers later presented histologic evidence of new bone, cementum, and functionally-oriented periodontal ligament fibers following application of demineralized freeze-dried bone allografts (DFDBAs) in human infrabony periodontal defects (IBDs) [3]. Since these landmark studies, multiple biologically active therapeutic agents have been developed to manipulate innate wound healing processes and induce bone and periodontal regeneration [4,5]. Most of these agents, known collectively as “biologics,” are commercially available proteins that stimulate recruitment, growth, differentiation, and metabolic activity of cells involved in regeneration [5]. Among biologics currently in widespread use, only autologous blood-derived products (ABPs) are patient derived [5,6].
The first ABP with significant dental application was platelet-rich plasma (PRP) [7-9]. This first-generation platelet concentrate was hampered by a complicated, time-consuming, and expensive chairside preparation involving the addition of an anticoagulant, two centrifugation cycles, and multiple containers [10]. Moreover, PRP delivered inconsistent efficacy in controlled clinical investigations [6,9]. In 2006, Choukroun introduced a simplified preparation protocol in which a blood sample without anticoagulant was centrifuged in a single cycle for 10 minutes at 3000 rpm [10]. The modifications initiated the second generation of plate concentrates—platelet-rich fibrin (PRF)—which offered superior handling properties and clinical performance [6,10]. Notably, ABP preparation protocols, including centrifugation parameters, continue to evolve. A recent review identified ten unique ABP preparations [11]. The temperature, magnitude and duration of force applied to the blood, addition of anticoagulant, and centrifugation tube material have the potential to alter the biological properties and clinical handling of the product [6,11].
Despite technological advances, questions regarding the clinical efficacy of biologics remain. Performance of each biologic must be assessed in the context of specific procedure types, and the available data are incomplete [5,6]. For example, evidence that a particular biologic accelerates soft tissue healing does not necessarily imply long-term clinical benefit or a favorable influence across a spectrum of procedures. Despite limitations in available research, a recent American Academy of Periodontology best evidence consensus statement concluded that ABPs significantly enhance clinical and radiographic outcomes following surgical treatment of IBDs, PRF producing superior outcomes in this context compared with enamel matrix derivative and PRP [6]. Furthermore, ABPs improve handling properties of bone biomaterials and possibly enhance mineralized tissue formation in alveolar ridge preservation/reconstruction (ARP/ARR) [6]. Multiple biologics, including ABPs, enhance initial soft tissue healing and may diminish complication risk following some surgical procedures [5,6].
Intuitively, ABPs, which are patient derived, should be safe for clinical use, and indeed, no ABP-related risks or detrimental effects have been reported [5,6]. Considering the consensus that ABPs add clinical benefit to conventional periodontal procedures and the common practice of intravenous (IV) access for moderate sedation in periodontics, routine use of ABPs appears attractive. However, in the periodontics literature, best practices for obtaining blood samples from peripheral lines have received minimal attention. The purpose of this report is to describe an armamentarium and protocol for safely obtaining blood samples for ABP preparation and demonstrate sample volumes typically needed in periodontics.
Materials And Methods
Patients in this report presented to the Department of Periodontics, Army Postgraduate Dental School (APDS), Postgraduate Dental College, Fort Eisenhower, Georgia, and completed an informed consent process involving verbal and written components. All patients received intravenous cannulation for moderate sedation and elected to provide blood samples for PRF preparation using the described protocol (Figures 1 and 2, Tables 1 and 2) [12-16]. In all cases, a single 20-gauge intravenous catheter was used for both blood collection and fluid/medication delivery [17-20]. In each case the blood was centrifuged at 1300 RPM for 14 minutes in sterile vacuum tubes.
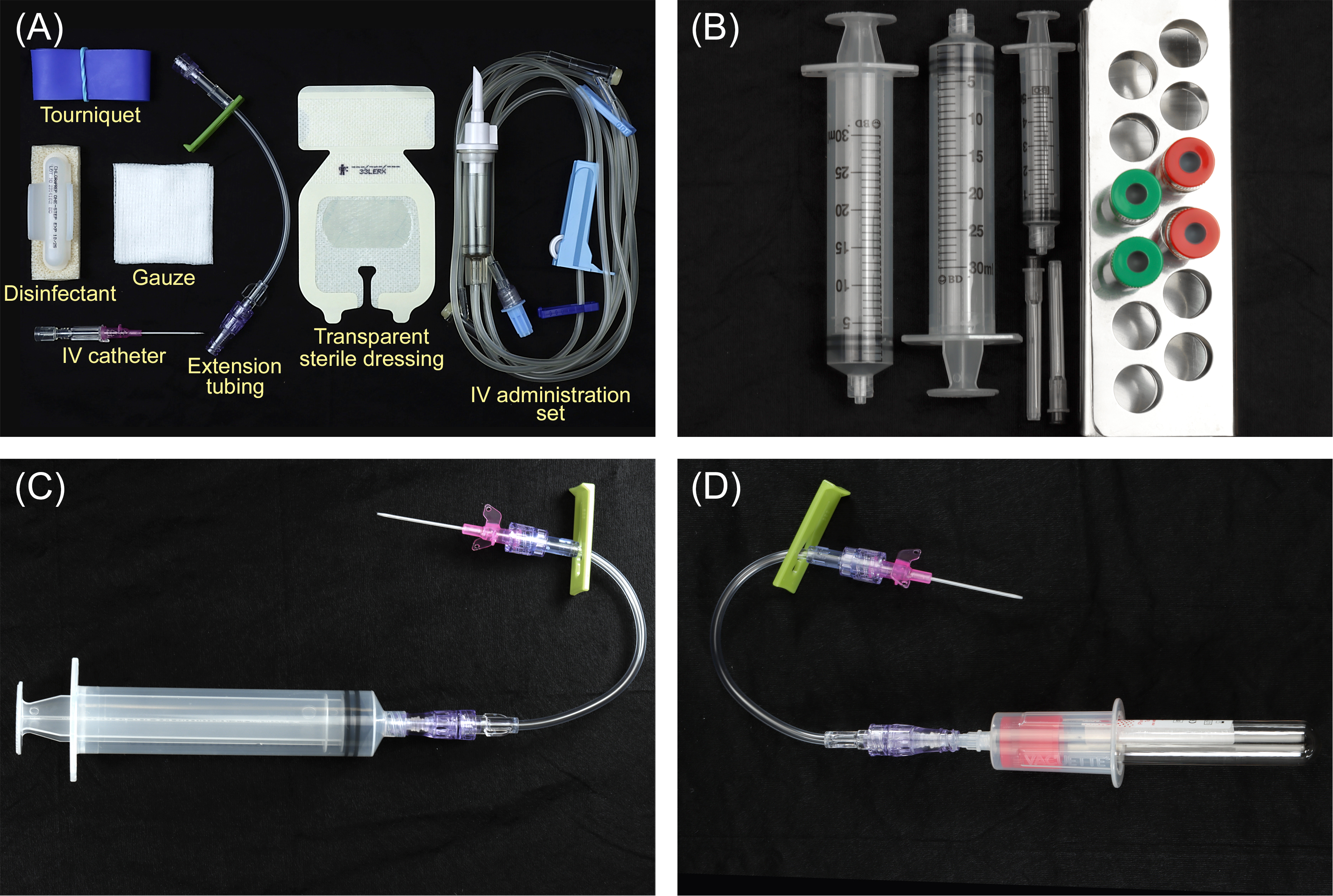
Figure 1: Armamentarium. (A) Tourniquet, skin disinfectant, intravenous (IV) catheter (20-guage or larger), lockable extension tubing to permit syringe connection, gauze, transparent sterile dressing, and an IV administration set (infusion tubing with injection port). (B) Green-capped plastic centrifuge tubes used to prepare liquid platelet-rich fibrin (PRF), which is commonly added to bone grafts and biomaterials. Red-capped glass centrifuge tubes are used to prepare PRF membranes. One or two 30-ml syringes are used to collect the blood. A 3- or 5-ml syringe is used to prime the extension tubing with blood prior to sample collection. (C) A 30-ml syringe is shown connected to the extension tubing and a 20-gauge IV catheter. (D) As an alternative to transferring blood from a 30-ml syringe to the centrifuge tubes, the centrifuge tubes can be filled directly using a vacutainer set-up. This method permits rapid collection of a small volume of blood but requires access to a large vein with adequate flow.

Figure 2: Blood collection for autologous blood product preparation. (A) A proximal tourniquet is applied, and a large vein in the antecubital fossa is cannulated with a 20-gauge or larger intravenous catheter. (B) The saline lock. The saline-primed intravenous (IV) administration set and extension tubing are connected to the catheter. Cannulation and patency are confirmed by observing proper saline flow without patient discomfort. (C) Fluids are held, the green lock on the extension tubing is activated, and the tubing is disconnected from the extension. A 5-ml syringe is connected. The extension tubing lock is released, and saline is removed from the extension tubing through gentle aspiration. The lock is re-applied, and the 5-ml syringe is removed. (D) A 30-ml syringe is used for blood collection.
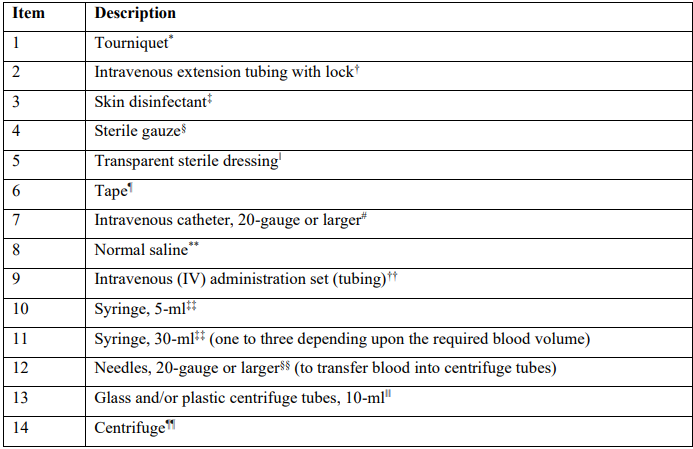
Table 1: Example blood collection armamentarium for autologous blood-derived product preparation
*Tourniquet, Medical Action Industries, Mechanicsville, VA
†IV extension set, Becton Dickinson, Franklin Lakes, NJ
‡Chloraprep, Becton Dickinson, Franklin Lakes, NJ
§Sterile gauze pad 2x2, Cardinal Health, Dublin, OH
‖Tegaderm, 3M, Saint Paul, MN
¶Transpore surgical tape, 3M, Saint Paul, MN
#Intravenous catheter, Braun, Bethlehem, PA
**0.9% Sodium chloride injection 500 ml, Baxter, Deerfield, IL
††Interlink system, Baxter, Deerfield, IL
‡‡BD syringe, Becton Dickinson, Franklin Lakes, NJ
§§BD PrecisionGlide hypodermic needle, Becton Dickinson, Franklin Lakes, NJ
‖‖PRF tubes, Dental Implant Technologies, Scottsdale, AZ
¶¶Duo Quattro Dental PRF Centrifuge, Dental Implant Technologies, Scottsdale, AZ
Case 1
In June of 2020, a healthy male aged 41 years presented for evaluation of bone loss at the distal surface of the right mandibular second molar. Probing depths measured 1-3 mm generally, with bleeding 8-mm pockets distal to tooth #31. Radiographic findings included vertical bone loss to the apical third of the distal root. The patient elected a bone replacement graft with guided tissue regeneration using PRF. A 60-ml blood sample was collected for ABP preparation. Liquid PRF was combined with a particulate freeze-dried bone allograft (FDBA) and autogenous bone scrapings, then placed in the defect. Two PRF membranes were layered over the FDBA. Wound closure for primary intention healing was achieved with dense polytetrafluoroethylene sutures. Early healing was uneventful. After 2 years, partial radiographic bone fill was noted, residual probing depths measuring 4-5 mm (Figures 3 and 4).
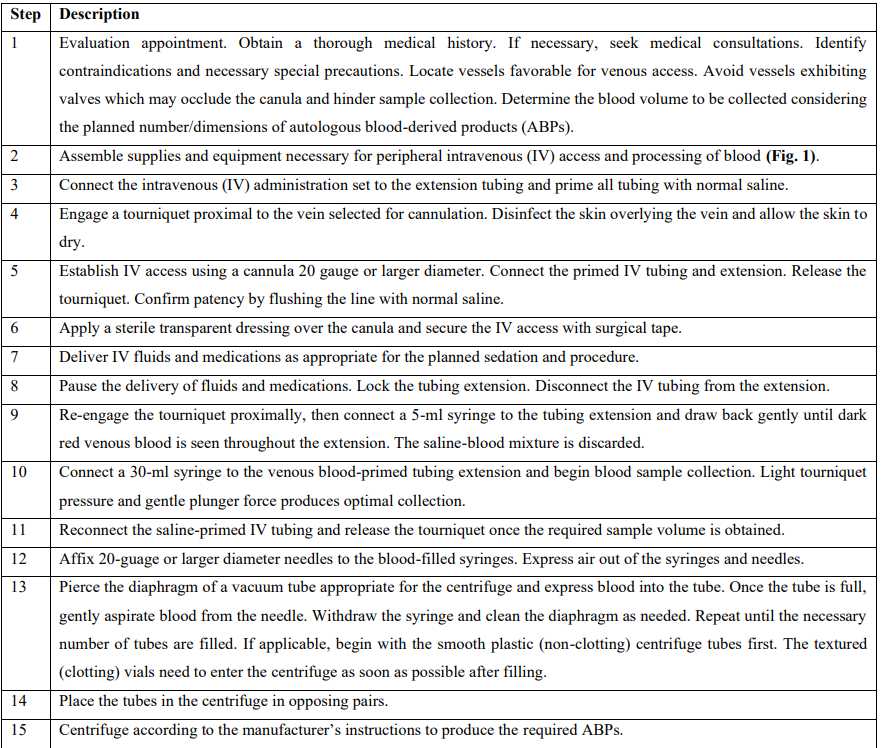
Table 2: Blood collection protocol for preparation of platelet-rich fibrin and other autologous blood-derived products
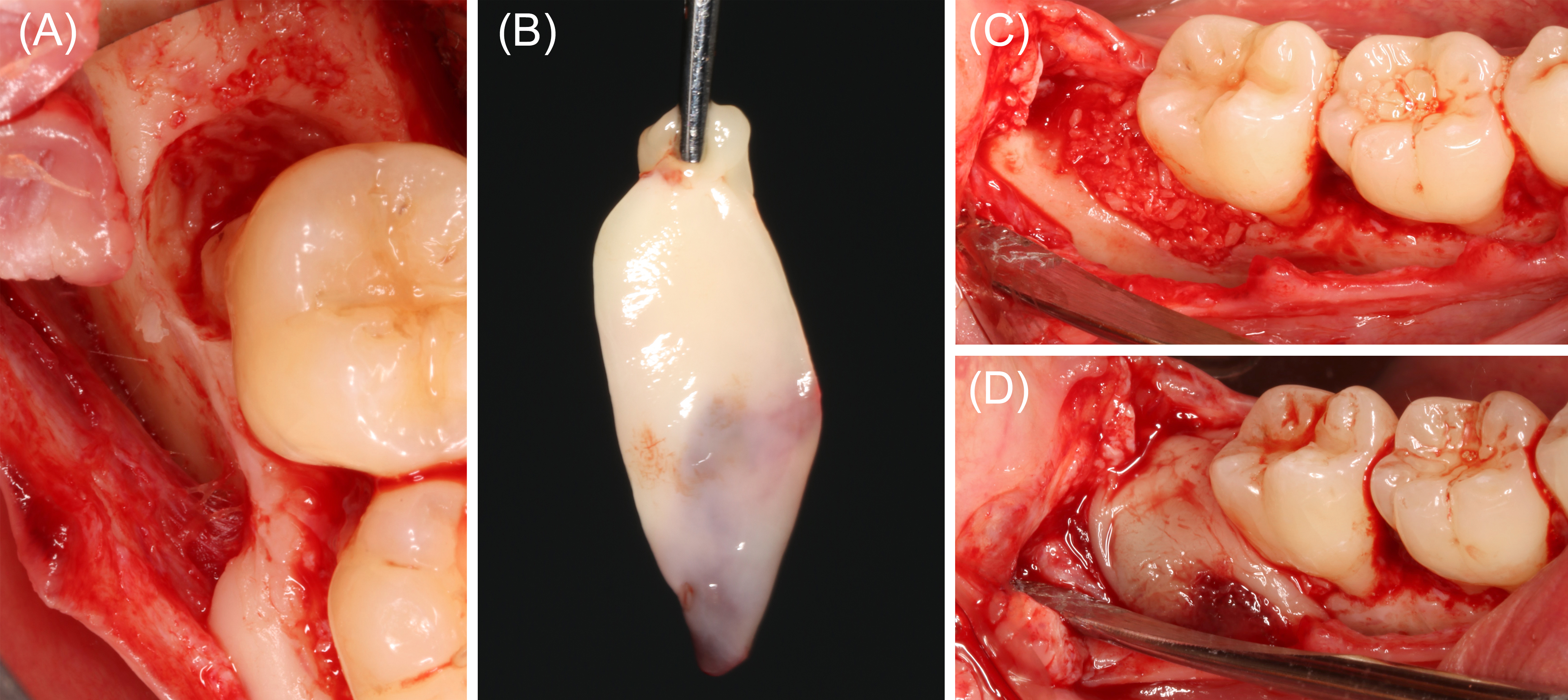
Figure 3: Case 1. Bone replacement graft and guided tissue regeneration, tooth #31 (60 ml of blood collected). (A) Tooth #31 exhibited a 3-wall intrabony defect at the distal surface, extending to the buccal furcation area. Calculus was noted on the root surface. After thorough debridement with an ultrasonic scaler, rotary instruments, and hand scalers, the root was treated with a 50-mg/ml tetracycline solution. (B) A 60-ml sample of blood was collected and transferred into plastic and glass centrifuge tubes, then centrifuged at 1300 rpm for 14 minutes. The product from the glass tubes were pressed to form platelet-rich fibrin (PRF) membranes. (C) Liquid PRF from the plastic tubes were combined with a particulate freeze-dried bone allograft and autogenous bone shavings, then applied in the periodontal defect. (D) PRF membranes were layered over the bone replacement graft prior to wound closure.
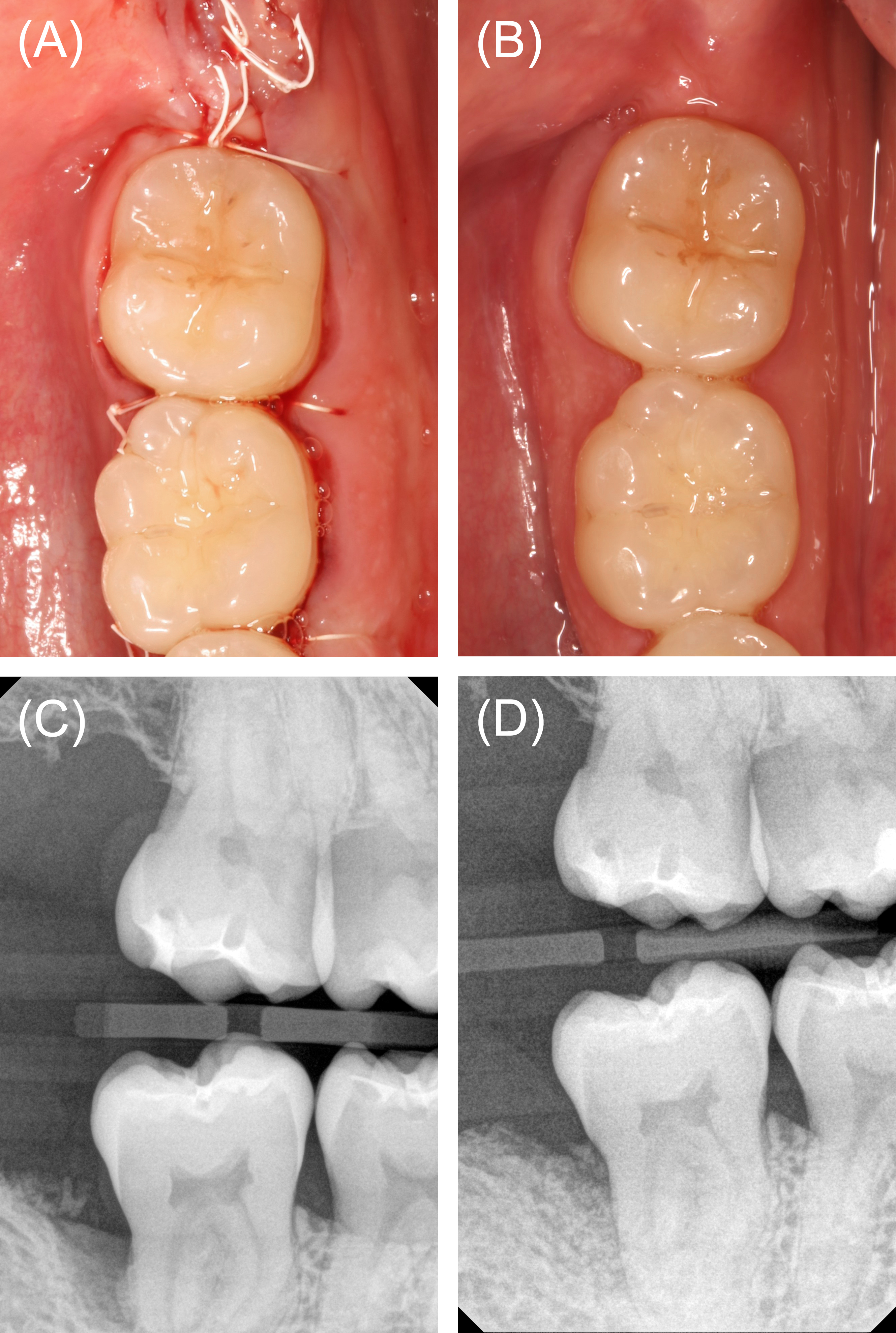
Figure 4: Case 1. Wound closure and follow-up. (A) Wound closure for primary intention healing was achieved with polytetrafluoroethylene sutures. (B) Clinical appearance at postoperative week 6. (C) Baseline vertical bite wing radiograph. (C) Vertical bite wing radiograph at postoperative month 24 demonstrating partial radiographic bone fill.
Case 2
In September of 2020, a healthy male aged 37 years was referred for extraction of non-restorable tooth #4, ARP, and implant placement. A 20-ml blood sample was drawn using the described protocol. Minimal buccal and palatal mucoperiosteal flaps were reflected to permit membrane stabilization, and tooth #4 was extracted. A PRF membrane was trimmed and condensed into the apical third of the socket, and a particulate FDBA filled the remainder of the alveolus. A second PRF membrane was placed over the socket orifice and stabilized under the buccal and palatal flaps using a crossed horizontal mattress suture# (Figures 5 and 6). After 4 months of healing, a 4 x 10 mm implant** was installed with insertion torque of 40 N-cm. An implant-supported crown was delivered 6 months following implant placement.
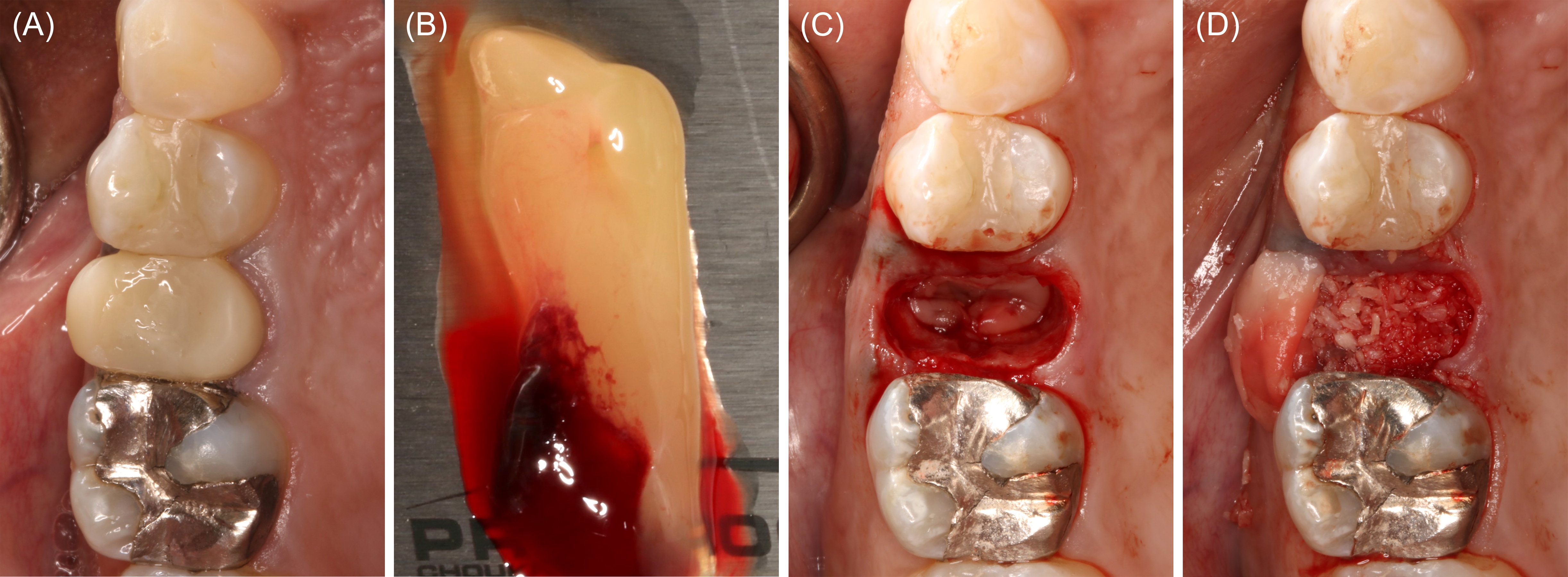
Figure 5: Extraction of tooth #4 with alveolar ridge preservation (20 ml of blood collected). (A) Baseline clinical appearance. (B) Platelet-rich fibrin (PRF) membranes prepared. (C) A PRF membrane was applied in the apical third of the extraction socket. (D) A particulate freeze-dried bone allograft was placed in the coronal two thirds of the alveolus.
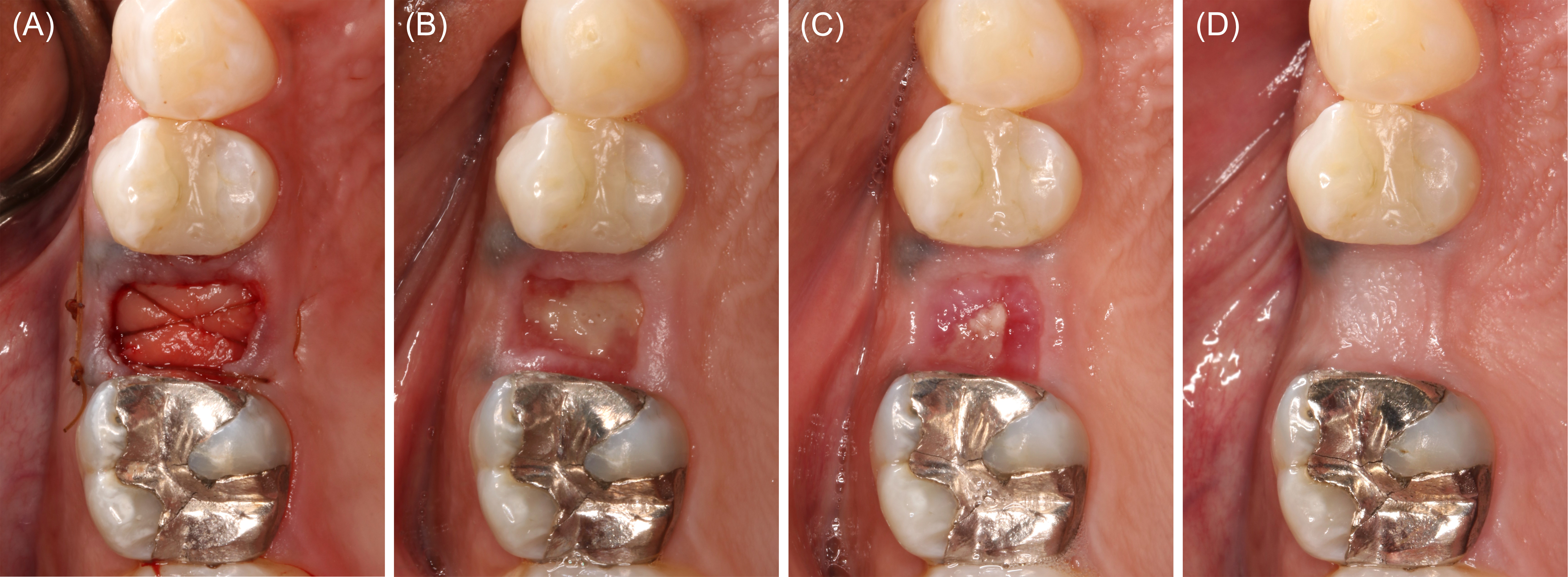
Figure 6: Case 2. Immediate postoperative appearance and healing. (A) A platelet-rich fibrin membrane was stabilized over the particulate freeze-dried bone allograft with chromic gut sutures. (B) Postoperative week 1. (C) Postoperative week 2. (D) Postoperative month 4.
Case 3
In July of 2022, a healthy male aged 57 years presented for implant evaluation. A 7.5-mm heart-shaped, low-density lesion near the midline, apical to the maxillary central incisors, was an incidental finding on the cone-beam computed tomography volume (Figure 7). An oral and maxillofacial radiology consult confirmed the lesion was consistent with nasopalatine duct cyst (NDC), and enucleation was recommended. A 60-ml blood sample was collected using the described technique. Aspiration of the lesion was negative. A full thickness palatal flap was reflected from tooth #5 to tooth #11. The thin, superficial bone partially covering the NDC was removed. The cyst was then enucleated and stored in formalin. The biopsy was interpreted by an oral pathologist who confirmed the NDC diagnosis. A particulate FDBA (1.2 ml) was mixed with liquid PRF. The PRF-allograft mixture was applied within the defect and covered with a PRF membrane (Figures 8 and 9). The palatal flap was approximated and stabilized with simple interrupted sutures.# The patient reported minimal discomfort limited to the first few postoperative days. At the 1-year follow-up assessment, the maxillary anterior appeared normal. No adverse effect of the surgery was appreciated.
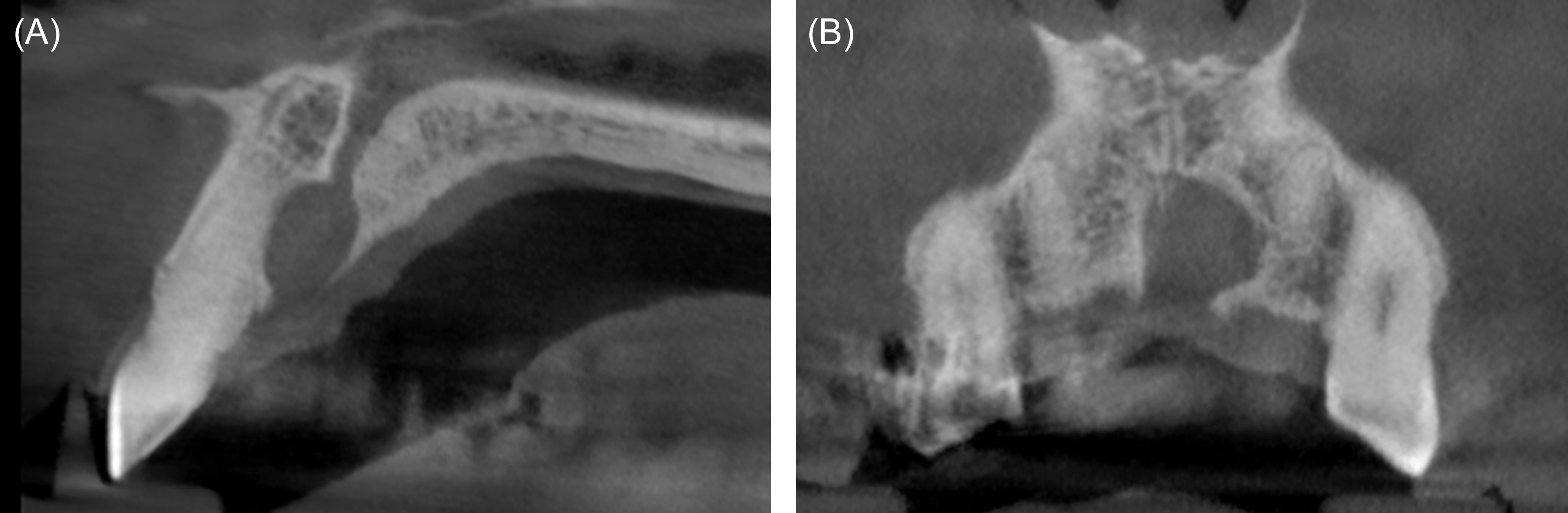
Figure 7: Case 3. Cone-beam computed tomography. (A) Parasagital view, tooth #9 area. (B) Custom view, maxillary anterior.
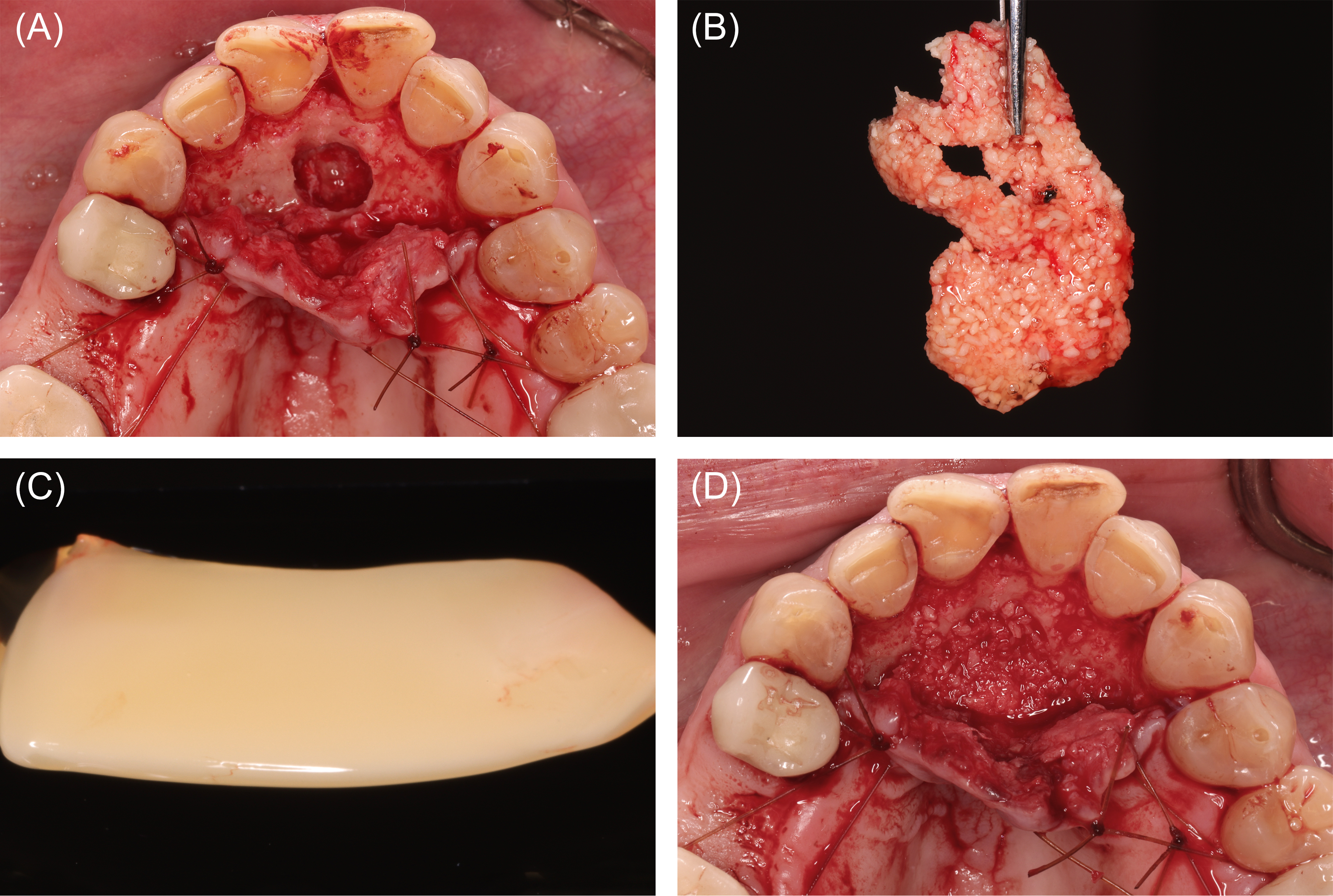
Figure 8: Case 3. Reconstruction following nasopalatine duct cyst (NDC) removal (60 ml of blood collected). (A) Defect following NDC removal. (B) Liquid platelet-rich fibrin (PRF) combined with a particulate freeze-dried bone allograft (FDBA). (C) PRF membranes prepared. (D) FDBA and liquid PRF applied.
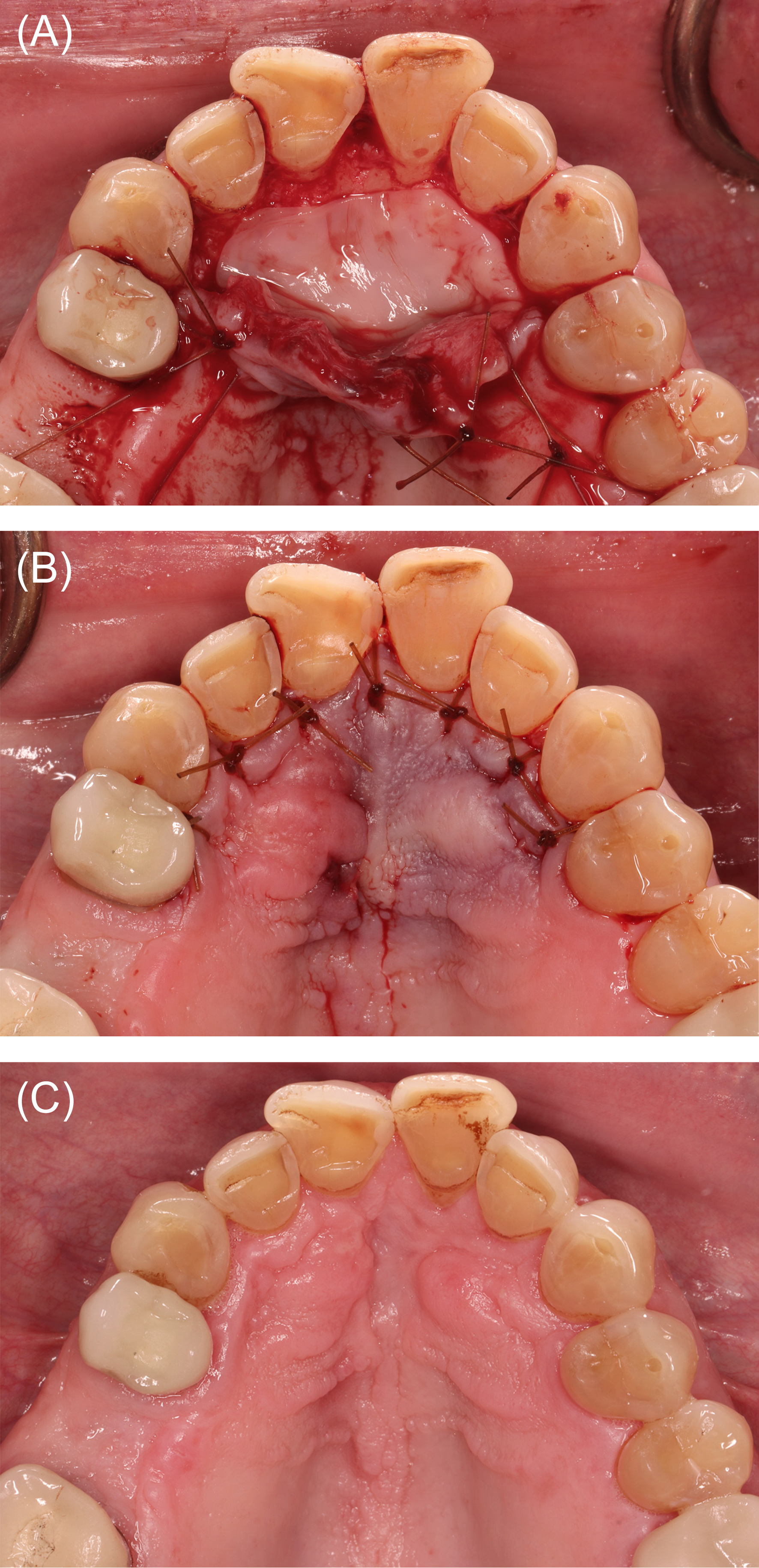
Figure 9: Case 3. Wound closure and healing. (A) Platelet-rich fibrin membranes layered over the freeze-dried bone allograft. (B) Wound closure. (C) Clinical appearance at postoperative month 4.
Case 4
In July of 2020, a healthy male aged 22 years missing tooth #4 presented for implant evaluation. A CBCT scan revealed sinus pneumatization and mucosal thickening in the tooth #4 area. The patient elected sinus elevation with delayed implant placement. A 60-ml blood sample was collected for ABP preparation as described. A full thickness buccal flap was reflected, and the maxillary sinus was accessed. The Schneiderian membrane was reflected to the medial wall, and no perforation was detected. A 2.4-ml particulate FDBA was hydrated in a tetracycline hydrochloride solution (100 mg/ml), rinsed thoroughly, combined with liquid PRF, and applied in the sinus. Wound closure for primary intention healing was achieved using PTFE sutures, and healing was uneventful. At postoperative month 6, a CBCT volume was acquired to permit virtual implant placement and surgical template fabrication. Seven months following sinus elevation, a 4 x 10 mm implant** was stabilized with insertion torque > 35 N-cm. A bone core biopsy was obtained at implant surgery (Figures 10 and 11) and processed for histological evaluation. The alveolar ridge appeared clinically and histologically favorable for implant placement (Figure 12). Healing was uneventful, and an implant-supported crown was delivered 6 months after implant placement.
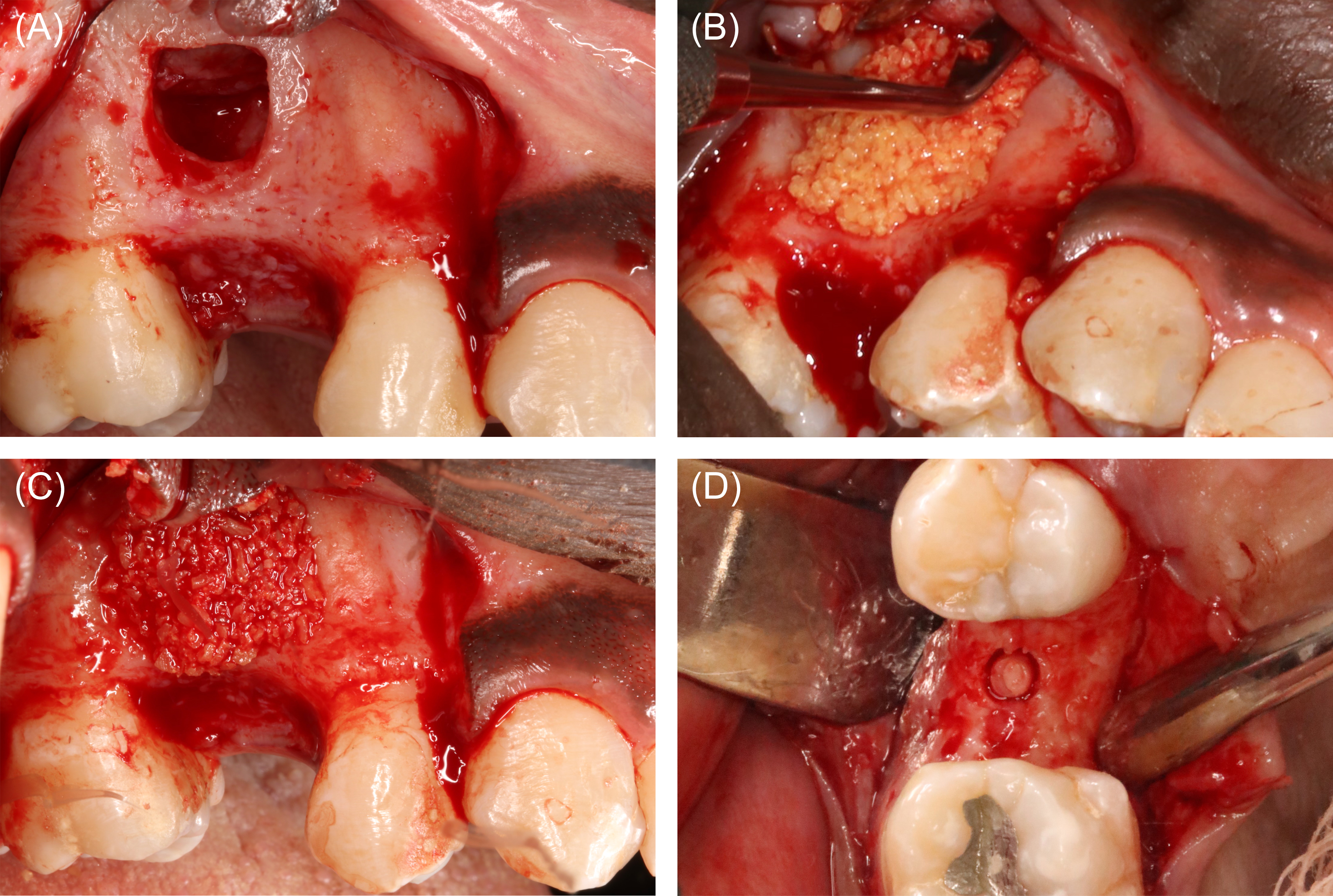
Figure 10: Case 4. Sinus elevation surgery (60 ml of blood collected). (A) Lateral window prepared to access the right maxillary sinus in the tooth #4 area. The Schneiderian membrane was reflected to the medial wall, and no membrane perforation was detected. (B) A 2.4-ml particulate freeze-dried bone allograft (FDBA) was hydrated in a 100-mg/ml tetracycline solution, and then rinsed 5 time with copious normal saline. Liquid platelet-rich fibrin (PRF) was then combined with the FDBA, and the mixture was applied in the antrum. (C) Liquid PRF and FDBA combination in place. (D) At implant surgery, a bone core biopsy was obtained to assess the quality of the bone produced.
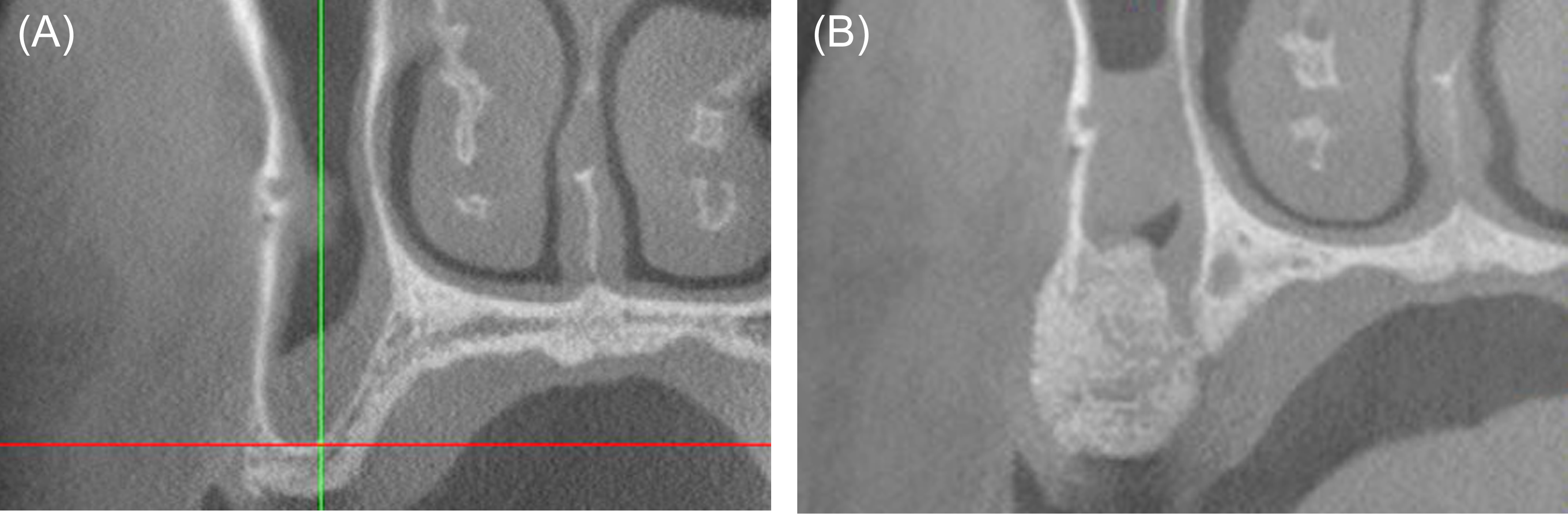
Figure 11: Case 4. Comparison of cone-beam computed tomography (CBCT) images. (A) Baseline CBCT image, cross-sectional view, tooth #4 area. (B) Cross-sectional CBCT image, tooth #4 area, postoperative month 6.
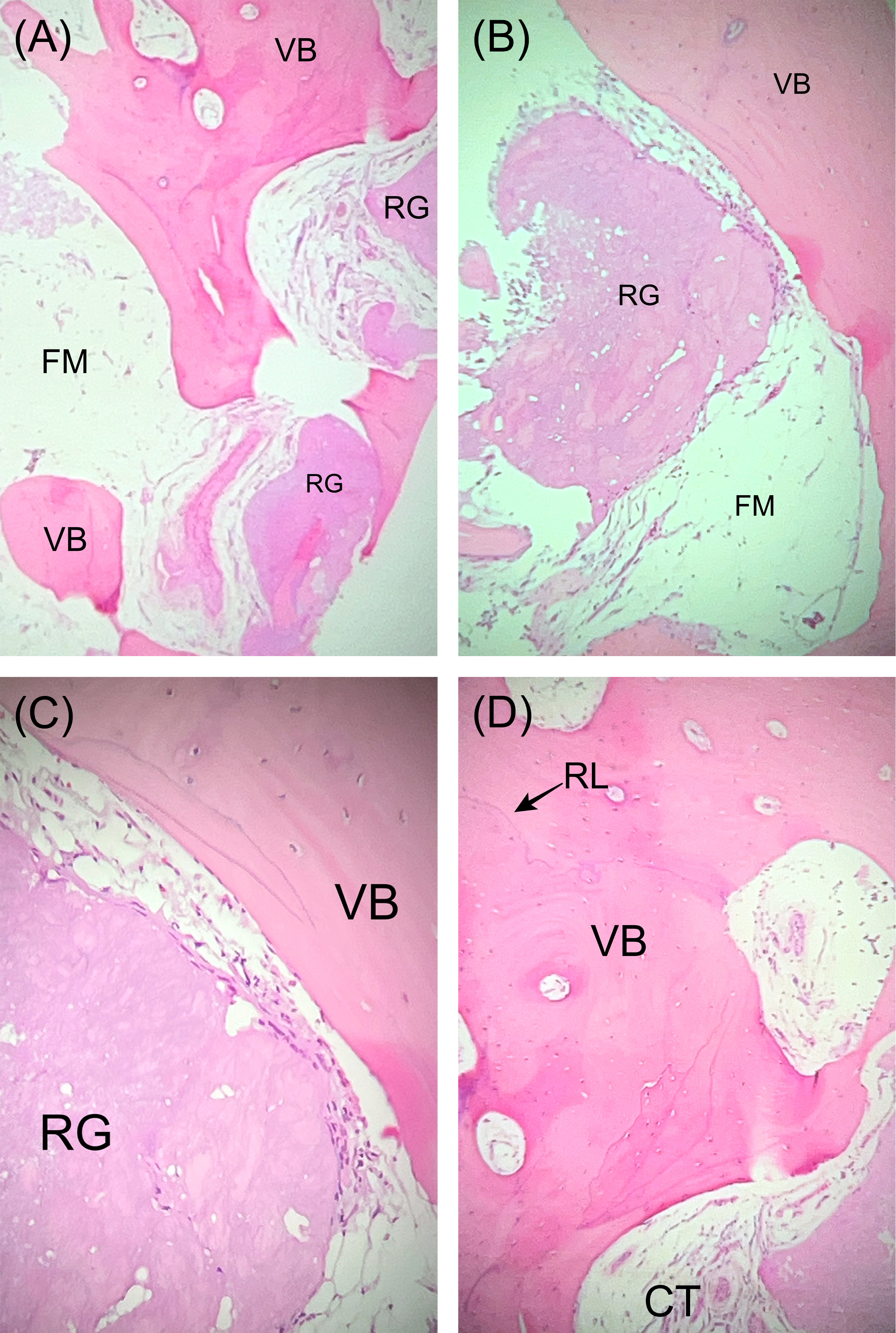
Figure 12: Case 4. Photomicrographs, hematoxylin and eosin staining. (A) Low-power (4x) view of the grafted site demonstrates vital bone (VB) and residual allograft particles (RG) admixed with fibro-fatty marrow (FM). (B) Medium-power (20x) view shows RG surrounded by newly formed VB and FM. (C) High-power (40x) view of VB juxtaposed with RG. (D) High-power (40x) view of viable bone (VB) with reversal lines (RL) shows evidence of remodeling in the new woven bone. Connective tissue (CT) is also present.
Case 5
In January of 2021, a healthy female aged 22 years complained of cold sensitivity from the left maxillary canine area. Tooth #11 exhibited 3 mm of gingival recession and a width of attached gingiva < 2 mm. The treatment plan included coronally advanced flap with subepithelial connective tissue graft (SCTG) and PRF. A 40-ml sample of blood was drawn for ABP preparation. Following full thickness facial flap reflection, the root was debrided with rotary, ultrasonic, and hand instruments. A periosteal releasing incision facilitated the coronal advancement of the flap, and the interproximal papillae were de-epithelialized. A SCTG was harvested from the left side of the palate using a single incision technique. A PRF membrane was placed in the palatal wound, and the margins were approximated with 5-0 chromic gut sutures.# The SCTG was submerged in liquid PRF, then stabilized at the cementoenamel junction of tooth #11. The coronally advanced flap was secured with sling†† and simple interrupted sutures.# Early healing was uneventful. Through one year of follow-up, no residual root exposure was noted. Esthetics were acceptable to the patient, and her chief complaint of hypersensitivity was adequately addressed (Figure 13).
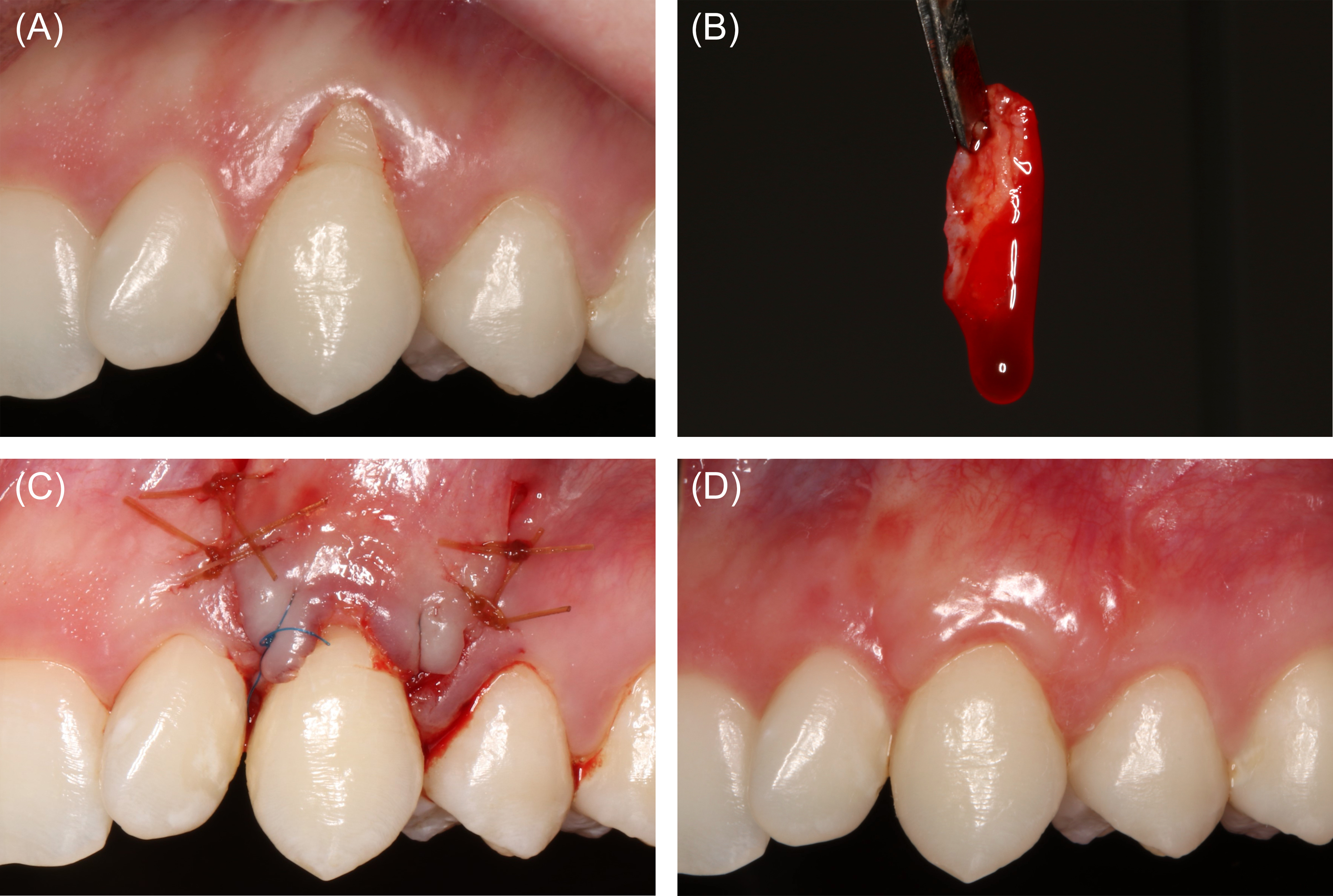
Figure 13: Case 5. Root coverage (40 ml of blood collected). (A) Preoperative view of the patients left maxillary canine with Recession Type 1A- and minimal attached gingiva. (B) A subepithelial connective tissue graft (SCTG) had been submerged in liquid platelet-rich fibrin. (C) Coronally advanced flap stabilized over the SCTG. (D) Clinical appearance at postoperative week 6.
Discussion
Specific guidelines on blood collection for ABP preparation do not exist. However, literature on blood collection for various medical purposes offers insight into best practices for periodontists. Blood sampling for screening and diagnostic testing ranks among the most common procedures in medicine, [21,22] and in hospitals, IV catheters are the most frequently used invasive medical devices [23-25]. Moreover, blood donation/banking for transfusion has been in widespread use since the 1940s. [26]. Admittedly, blood volumes safely obtainable during donation may have minimal relevance for patients undergoing surgical procedures. Typical blood donors are not subject to surgery-related stress and do not experience blood loss from a surgical wound. Nevertheless, the World Health Organization recommends limiting donations to 13% of total blood volume (TBV), which is known to correlate with body mass [27,28]. A typical 70-kg adult maintains about 5600 ml (5.9 quarts) of blood [27,28]. In perspective, routine blood donations range between 350 and 500 ml in volume [27]—7- to 10-fold higher than the average volume utilized in cases presented here.
Recommended total blood draw volume limits for screening, diagnostic testing, and other purposes have been defined for healthy and unhealthy individuals. For healthy patients, the maximum recommended sample volume within a 24-hour period is 3% of TBV (≈ 168 ml for a 70-kg adult) [27]. This limit is reduced to 2.5% of TBV (≈ 140 ml for a 70-kg adult) for compromised individuals [27]. Blood volumes collected for ABP preparation described in this report were substantially less than half of this maximum. At most institutions, maximum allowable blood sample volumes for research purposes mirror these clinical limitations [29,30].
Applying a saline lock (Figure 2) allows clinicians flexibility in the timing of blood collection. Preparation time required to produce an ABP varies by product type. Accordingly, clinicians may choose to collect blood prior to initiating surgery or pause intraoperatively to obtain the sample. In addition to deciding upon the blood draw timing, practitioners must select the number and type of centrifuge tubes. The tube choice depends upon the scope of the procedure and the ABP(s) required. Smooth plastic centrifuge tubes do not initiate clotting and are selected when liquid PRF is desired. In contrast, glass tubes do induce clotting and are chosen when plug- or membrane-formed PRF is needed. In many cases (Figures 3,4,7-9), practitioners utilize a combination of plastic and glass tubes to prepare multiple ABP types.
Although not specifically written for outpatient/ambulatory care, Infusion Therapy Standards of Practice, particularly those aiming for prevention of complications, are meant to be adapted and applied in any setting where infusion therapy is administered [24,31]. Peripheral IV catheter placement has been associated with adverse events such as phlebitis, local infiltration/extravasation, loss of patency, dislodgement, and infection [23,24,31-33]. Phlebitis is a poorly defined term, and consensus regarding identification and management of this complication is lacking. Thus, the incidence of phlebitis varies widely across studies—from <1% to 100% [33]. Phlebitis occurrence has been attributed to mechanical, chemical, and infective factors [23]. Rapid infusion and movement of the catheter intravenously may induce phlebitis by generating shear stress at the vessel wall [23]. Utilizing a catheter excessively large relative to the selected vein may contribute to mechanical phlebitis [13,23], especially in the presence of shear stress related to blood collection. The catheter should be free-floating within the lumen of the vessel [13,23]. Thus, periodontists may prefer a relatively large-diameter vein in the antecubital fossa—typically the basilic vein, median cubital vein, or cephalic vein—when planning blood collection. Chemical phlebitis relates to properties of medications delivered through IV catheters, particularly osmolality, pH, and solubility [23]. Among sedatives commonly used by periodontists, diazepam is associated with a comparatively high phlebitis rate [34].
Interestingly, incidence of infiltration and extravasation appears substantially higher in emergency departments (ED) compared with other hospital divisions [33]. This excess incidence may be attributable to use of IV catheters to draw blood, large-bore catheters, and high-volume fluid delivery in EDs [33]. Nevertheless, ability to collect blood through the same catheter used for fluid and medication delivery is a well-supported practicality that increases the convenience of ABP preparation [17-20]. No instances of infiltration/extravasation have occurred while applying the described protocol in the APDS from 2020 through 2023.
To maintain patency, it is recommended to flush peripheral IV catheters with 2 to 10 ml of normal saline or another appropriate fluid after each IV medication administered, or in the hospital setting, every 4 to 12 hours [12-14]. To manage an occluded catheter, practitioners should gently aspirate with a 5-ml syringe, then flush with 2 to 10 ml of normal saline. If resistance is encountered when performing the saline flush, the catheter should be removed, and a new catheter should be placed at another site [24,35].
Peripheral IV catheter dislodgement is generally preventable by properly securing the IV line with tape, thus countering forces that would remove the catheter from the vein. Dislodgement is a nuisance because it may interrupt sedation, surgery, and blood collection but also a threat to the practitioner’s ability to deliver reversal agents and other emergency medications. Thus, securing the IV line after catheter placement should be routine practice. Infection—including infective phlebitis—is considered a rare complication of peripheral IV catheter placement when Aseptic Non-Touch Technique (ANTT) is observed [23,31]. The skin overlying the site should be thoroughly disinfected before catheter placement, and a transparent sterile dressing should protect the site of venous access intraoperatively [23,24].
Conclusion
For clinicians providing moderate IV sedation, barriers to routine clinical ABP use are minimal. Blood volumes required for ABP preparation amount to a fraction of published recommended total blood draw volume limits. The blood collection method described in this report, which is consistent with published standards of practice, necessitates few additional steps and supplies for practitioners already placing peripheral IV catheters. By incorporating routine ABP use into their practices, clinicians may consistently accelerate wound healing in a variety of procedure types, and for some indications, enhance clinical outcomes.
Footnotes
¶Cytoplast, Osteogenics, Lubbock, TX
#Chromic Gut Monofilament Suture, Ethicon, Raritan, NJ
**Osseotite Certain, ZimVie, Westminster, CO
††Polypropylene Suture, Ethicon, Raritan, NJ
Disclosure statement
The views expressed in this manuscript are those of the authors and do not necessarily reflect the official policy of the United States Government, the Department of Defense, the Defense Health Agency, or Uniformed Services University.
Funding source: The Defense Health Agency funded this research entirely. The authors received no extramural funding for this work.
Author contribution statement: All authors have contributed substantially to conceptualization of the article, writing the original draft, critical review, and editing. All authors have approved the final version of the manuscript.
Conflict of interest statement
The authors report no financial, economic, or professional interests that may have influenced the design, execution, or presentation of this work.
References
- Urist MR. (1965) Bone: formation by autoinduction. Science. 150(3698): 893-899. [PubMed.]
- Bang G, Urist MR. (1967) Bone induction in excavation chambers in matrix of decalcified dentin. Arch Surg. 94(6): 781-789. [PubMed.]
- Bowers GM, Chadroff B, Carnevale R, et al. (1989) Histologic evaluation of new attachment apparatus formation in humans. Part III. J Periodontol. 60(12): 683-693. [PubMed.]
- Susin C, Wikesjö UM. (2013) Regenerative periodontal therapy: 30 years of lessons learned and unlearned. Periodontol 2000. 62(1): 232-242. [PubMed.]
- Suárez-López Del Amo F, Monje A. (2022) Efficacy of biologics for alveolar ridge preservation/reconstruction and implant site development: An American Academy of Periodontology best evidence systematic review. J Periodontol. 93(12): 1827-1847. [PubMed.]
- Avila-Ortiz G, Ambruster J, Barootchi S, et al. (2022) American Academy of Periodontology best evidence consensus statement on the use of biologics in clinical practice. J Periodontol. 93(12): 1763-1770. [PubMed.]
- Carlson NE, Roach RB Jr. (2002) Platelet-rich plasma: clinical applications in dentistry. J Am Dent Assoc. 133(10): 1383-1386. [PubMed.]
- Marx RE. (2001) Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 10(4): 225-228. [Ref.]
- Pocaterra A, Caruso S, Bernardi S, et al. (2016) Effectiveness of platelet-rich plasma as an adjunctive material to bone graft: a systematic review and meta-analysis of randomized controlled clinical trials. Int J Oral Maxillofac Surg. 45(8): 1027-1034. [PubMed.]
- Dohan DM, Choukroun J, Diss A, et al. (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 101(3): e37-44. [PubMed.]
- Palaiologou A, Keeling F. (2022) Autologous blood products: Usage and preparation protocols. Clin Adv Periodontics. 12(4): 287-293. [PubMed.]
- Fernandez RS, Griffiths RD, Murie P. (2003) Peripheral venous catheters: a review of current practices. J Infus Nurs. 26(6): 388-392. [PubMed.]
- Frank RL, Wolfson AB, Ganetsky M. (2023) Peripheral venous access in adults. In: Post T, ed. UpToDate. [Ref.]
- Hadaway LC. (1998) Major thrombotic and nonthrombotic complications. Loss of patency. J Intraven Nurs. 21(5 Suppl): S143-S160. [PubMed.]
- Halm MA, Gleaves M. (2009) Obtaining blood samples from peripheral intravenous catheters: best practice? Am J Crit Care. 18(5): 474-478. [PubMed.]
- Sotnikova C, Fasoi G, Efstathiou F, et al. (2020) The efficacy of normal saline (N/S 0.9%) versus heparin solution in maintaining patency of peripheral venous catheter and avoiding complications: a systematic review. Mater Sociomed. 32(1): 29-34. [PubMed.]
- Dietrich H. (2014) One poke or two: can intravenous catheters provide an acceptable blood sample? A data set presentation, review of previous data sets, and discussion. J Emerg Nurs. 40(6): 575-578. [PubMed.]
- Hadaway L. (2003) Can I stop a drug infusion to draw blood? Nursing. 33(5): 14. [PubMed.]
- Lesser FD, Lanham DA, Davis D. (2020) Blood sampled from existing peripheral IV cannulae yields results equivalent to venepuncture: a systematic review. JRSM Open. 11(5): 2054270419894817. [PubMed.]
- Himberger JR, Himberger LC. (2001) Accuracy of drawing blood through infusing intravenous lines. Heart Lung. 30(1): 66-73. [PubMed.]
- Quinn JG, Tansey EA, Johnson CD, Roe SM, Montgomery LE. (2016) Blood: tests used to assess the physiological and immunological properties of blood. Adv Physiol Educ. 40(2): 165-175. [PubMed.]
- Czoski-Murray C, Lloyd Jones M, McCabe C, et al. (2012) What is the value of routinely testing full blood count, electrolytes and urea, and pulmonary function tests before elective surgery in patients with no apparent clinical indication and in subgroups of patients with common comorbidities: a systematic review of the clinical and cost-effective literature. Health Technol Assess. 16(50): i-xvi, 1-159. [PubMed.]
- Nickel B. (2019) Peripheral Intravenous Administration of high-risk infusions in critical care: A risk-benefit analysis. Crit Care Nurse. 39(6): 16-28. [PubMed.]
- Gorski LA. (2017) The 2016 infusion therapy standards of practice. Home Healthc Now. 35(1): 10-18. [PubMed.]
- Zingg W, Barton A, Bitmead J, Eggimann P, Pujol M, et al. (2023) Best practice in the use of peripheral venous catheters: A scoping review and expert consensus. Infect Prev Pract. 5(2): 100271. [PubMed.]
- Learoyd P. (2012) The history of blood transfusion prior to the 20th century--part 1. Transfus Med. 22(5): 308-314. [PubMed.]
- World Health Organization. (2012) Blood Donor Selection: Guidelines on Assessing Donor Suitability for Blood Donation. Geneva: World Health Organization. [Ref.]
- Kiechle FL. (2017) So You’re Going to Collect a Blood Specimen: An Introduction to Phlebotomy. 15th ed. CAP Press. PUB225. [Ref.]
- University of Pennsylvania Human Research Protections Program. (2023) Maximum allowable blood draw volumes. [Ref.]
- Peplow C, Assfalg R, Beyerlein A, et al. (2019) Blood draws up to 3% of blood volume in clinical trials are safe in children. Acta Paediatr. 108(5): 940-944. [PubMed.]
- Gorski LA, Hadaway L, Hagle ME, et al. (2021) Infusion Therapy Standards of Practice, 8th Edition. J Infus Nurs. 44(Suppl 1): S1-S224. [PubMed.]
- Helm RE, Klausner JD, Klemperer JD, Flint LM, Huang E. (2015) Accepted but unacceptable: peripheral IV catheter failure. J Infus Nurs. 38(3): 189-203. [PubMed.]
- Marsh N, Webster J, Ullman AJ, et al. (2020) Peripheral intravenous catheter non-infectious complications in adults: A systematic review and meta-analysis. J Adv Nurs. 76(12): 3346-3362. [PubMed.]
- Litchfield NB. (1983) Venous complications of intravenous diazepam. J Oral Maxillofac Surg. 41(11): 701-705. [PubMed.]
- Barrus DH, Danek G. (1987) Should you irrigate an occluded i.v. line? Nursing. 17(3): 63-64. [PubMed.]