>Corresponding Author : Neeta S Roy
>Article Type : Review Article
>Volume : 2 | Issue : 2
>Received Date : 13 July, 2022
>Accepted Date : 24 July, 2022
>Published Date : 04 Aug, 2022
>DOI : https://doi.org/10.54289/JORVC2200108
>Citation : Simpson CL, Asbell PA and Roy NS. (2022) Review of the Role of Oxidative Stress in Ocular Surface Disease and Use of MDA and 4-HNE in Tears as Potential Markers. J Ophthalmic Res Vis Care 2(2): doi https://doi.org/10.54289/JORVC2200108
>Copyright : © 2022 Simpson CL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Review Article | Open Access
1Department of Ophthalmology, Hamilton Eye Institute, University of Tennessee Health Science Center, 930 Madison Avenue, Memphis, TN, USA
2Department of Ophthalmology, Weill Cornell Medicine, New York, NY10065
*Corresponding author: Neeta S Roy, Department of Ophthalmology, Weill Cornell Medicine, New York, NY10065
Abstract
This article addresses the possible use of malondialdehyde (MDA) and 4-hydroxy-2-nonenal (4-HNE) as biomarkers in the tears for diagnosis, determining disease severity, and monitoring the effect of treatment for dry eye disease (DED), a disease which is highly prevalent and heterogeneous with an incompletely understood pathogenesis and limited therapeutic options. The current diagnosis and classification of severity of DED is a mostly subjective process with no consistent objective markers of disease, so the identification of novel biomarkers could improve patient care as well as lead to a better understanding of the disease pathogenesis and discovery of new treatments. This review is the first to compare the results of studies of markers of oxidative stress MDA and 4-HNE in DED and other ocular diseases to more comprehensively explore the potential for the use of MDA and 4-HNE as biomarkers for DED. The role of oxidative stress in DED is reviewed and then the evidence of their association with DED and other ocular diseases. Overall, previous studies indicate a promising potential for the use of tear MDA and 4-HNE as biomarkers for DED that should be explored with further research.
Abbreviations: MDA: Malondialdehyde, HNE: Hydroxy-2-Nonenal, DED: Dry Eye Disease, ROS: Reactive Oxygen Species, PUFA: Polyunsaturated Fatty Acids, L: Lipid Radical, LOO: Lipid Peroxy Radical, LOOH: Lipid Peroxidation Are Lipid Hydroperoxides, AA: Arachidonic Acid, TBA: Thiobarbituric Acid, HPLC: High-Performance Liquid Chromatography, HCEC: Human Corneal Epithelial Cells, HEL: Hexanoyl-Lysine, GCD: Granular Corneal Dystrophy, CSCR: Central Serous Chorioretinopathy, SS: Sjögren Syndrome, PS: Pterostilbene, PACG: Primary Angle-Closure Glaucoma, AKC: Atopic Keratoconjunctivitis, VF: Visual Field, KC: Keratoconus, wAMD: Wet Age Related Macular Degeneration, dAMD: Dry Age Related Macular Degeneration, S: Supplementation
Oxidative stress in ocular surface disease
The ocular surface is particularly susceptible to damage from oxidative stress. Oxidative stress is defined as a state in which more pro-oxidants exist in a cell than antioxidants, creating a potentially damaging imbalance [1] with excess reactive oxygen species (ROS), which are generated by partial reduction of oxygen in normal metabolic processes of the cell or by exogenous stressors [2]. When ROS are produced in excess of the antioxidants’ capability to rapidly catabolize them, oxidative stress can harm the cell via nonspecific free radical attack of biomolecules [1]. The ocular surface is at particular risk of high levels of ROS from exogenous sources due to its exposure to atmospheric oxygen and environmental stressors like smoke, pollutants, and UV radiation. Tear fluid contains protective antioxidants including ascorbic acid, lactoferrin, uric acid, and cysteine, but when too much ROS is generated, pro-oxidants overwhelm the antioxidants’ protective capacity and create a state of oxidative stress [2]. This oxidative stress can then feedback on itself by inducing stress signalling that activates an inflammatory response that can cause further damage and dryness of the eye as immune cells invade the lacrimal gland, meibomian gland, and ocular surface [3]. In addition to activating the inflammatory response, ROS can themselves damage the ocular surface [4]. The ocular surface is made up of a specialized stratified epithelium with a high density of mucin-producing goblet cells, and resident immune cells [5]. The epithelium and immune cells, including natural killer cells, dendritic cells, macrophages, CD4+ and CD8+ T cells, function as antimicrobial defence for the exposed mucosa of the ocular surface [5].
Oxidative stress has been found to play a role in ocular conditions, including various diseases of the ocular surface [6]. Oxidative stress has been studied and found to likely play a role in the pathogenesis of pterygia [7] and granular corneal dystrophy [8] by increasing signalling pathways for cellular growth. In Fuchs endothelial corneal dystrophy, oxidative stress causes corneal cell apoptosis [9]. Oxidative stress has been proposed as one of the key contributors in the pathogenesis of DED via inflammatory signalling and direct damage to the ocular surface [3 5 10]. Stress signalling from the epithelium and inflammation driven by the immune cells, particularly autoimmune CD4+ T cells and dendritic cells, has been shown to increase oxidative stress and is thought to contribute to the pathogenesis of dry eye [5]. ROS have also been shown to cause metaplasia and goblet cell loss in the ocular surface [5] and damage the tear lipid layer [4], both of which directly inhibit the ability of the eye to maintain a moist surface. A new study has shown antioxidant therapy to be effective at reducing the amount of ROS as well as the amount of inflammatory cytokines and chemokines, macrophage proinflammatory transformation, and cell apoptosis [11]. With promising antioxidant therapies being developed, biomarkers that could potentially measure the clinical efficacy of such treatments could play a crucial role in revolutionizing the treatment of DED.
Need for objective markers for eye disease, such as DED
Dry eye disease (DED) is a highly prevalent multifactorial disease that results in symptoms of discomfort, visual disturbance, and tear film breakup with potential damage to the ocular surface [10]. The global prevalence of DED has been reported to be between 5 and 35% [12]. DED can have an adverse effect on overall quality of life by causing discomfort and difficulty performing everyday activities, which translates into significant economic burden, with the total annual cost for the management of DED estimated to be $3.84 billion in the United States [12].
DED can occur when there is insufficient tear production and secretion or when there is excessive evaporation of the tear film at the ocular surface, causing an unstable and hyperosmolar tear film [13]. Changes in tear film can be initiated by numerous intrinsic or extrinsic factors such as systemic disease, infection, or environmental changes, leading to an inflammatory cycle that is a known feature of DED [5]. In this inflammatory cycle, dryness of the corneal epithelium stimulates signalling pathways that activate immune cell responses that include the production of matrix metalloprotease, recruitment of inflammatory cells, maturation of dendritic cells, and eventual T-cell response. The immune response damages the corneal epithelium and causes dysfunction and death of conjunctival goblet cells; these changes further destabilize the tear film and amplify the initial problem in a vicious cycle [3 5 10]. Inflammation has been shown to induce the production reactive oxygen species (ROS) which causes oxidative stress resulting in cell damage, including the peroxidation of the cell membrane lipids [14]. Malondialdehyde (MDA) and 4-hydroxy-2-nonenal (4-HNE) are two lipid peroxidation products of oxidative injury [14]. Their presence has specifically been demonstrated in tears from patients of DED [15 16].
There is no definitive diagnostic test for DED. Current diagnosis and assessment of DED involves largely subjective measures that can be difficult to accurately measure or quantitatively score. Multiple clinical assessments do exist to examine the ocular surface and tear functional unit, but the same assessments are not uniformly performed in a DED workup and threshold values for distinguishing pathologic DED are not clearly defined [15]. Furthermore, the administration of tests like inserting the Schirmer test strip can influence the amount or type of tears secreted, and other tests like the corneal staining tests use scoring systems that are subject to observer bias by the clinician [15]. While these tests may generally allow for the diagnosis of DED, their inconsistency makes determining disease severity and effectiveness of treatment imprecise both in the clinic and in clinical trials [16]. As defined by the FDA-NIH Joint Leadership Council biomarkers are “a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or intervention [they] may include molecular, histologic, radiographic, or physiologic characteristics” [17]. Biomarkers are currently being used in a number of diseases like cystic fibrosis (sweat chloride), diabetes (HbA1c), and chronic kidney disease (GFR) and are being developed for others [17]. Biomarkers be used for accurate diagnosis, classifying severity, and tracking the effectiveness of experimental treatments because they provide an objectively quantifiable metric for disease [18]. In this regards, tear levels of MDA and 4-HNE have the potential to be objective measurable biomarkers of DED to significantly improve the prediction and diagnosis of DED as well as the monitoring of patient responses to investigative therapeutic treatments.
MDA and 4-HNE as markers of oxidative stress
In measuring levels of oxidative stress, the direct measurement of ROS is often difficult due to the short half-life of ROS, so another approach is to measure stable downstream products of oxidative stress like MDA and 4-HNE [19-20]. Many markers of oxidative stress have been proposed based on ROS-induced modification of existing cell structures, including oxidation products of lipids (MDA, 4-HNE, alkenals, F2-IsoPs), DNA (8oxodG, 5-chlorocytosine, 5-chlorouracil), proteins (AGEs, carbonils, 3-nitro-tyrosine, Cl-tyrosine) [19]. MDA and 4-HNE are two of the most widely studied markers of oxidative stress [19], with a review of markers of oxidative stress rating MDA and 4-HNE as having the highest level evidence based on their presence in meta-analyses and large prospective studies compared to other markers [18]. They have been identified as the most cytotoxic breakdown products generated from lipid peroxidation [21], making MDA and 4-HNE possibly more clinically relevant markers than other products of oxidative stress. Both MDA and 4-HNE are easily measured; however, some methods of detection have been proposed to be too unspecific and prone to artefacts [18 19].
Lipid peroxidation is the oxidative deterioration of polyunsaturated fatty acids (PUFAs) [14] (figure 1). The double bonds of PUFAs weaken C-H bonds on adjacent carbon atoms, making them more susceptible to peroxidation via allylic hydrogen atom abstraction or reactive species addition [22]. The process of lipid peroxidation occurs via a process of initiation, propagation, and termination. Initiation most commonly occurs when a hydroxyl radical or hydroperoxyl radical abstract allylic hydrogens from PUFAs to form a carbon-centered lipid radical (L•). During propagation, the lipid radical reacts with oxygen to form a lipid peroxy radical (LOO•) which can then oxidize another PUFA, creating a chain reaction until termination occurs via an antioxidant pathway [14]. Peroxidized lipids have similar effects in the cell as ROS, with low levels acting as signalling molecules that can upregulate an adaptive stress response via stimulation of antioxidant systems and intermediate and high levels inducing cell damage and apoptosis [22].
While the main products of lipid peroxidation are lipid hydroperoxides (LOOH), aldehydes including malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) are secondary products of lipid peroxidation of omega-6 fatty acids [14]. Arachidonic acid (AA) is the most common omega-6 fatty acid in the cell and therefore the main precursor of MDA and 4-HNE [14]. MDA is formed when intermediate lipid free radicals cyclize to form bicycle endoperoxides which are then cleaved to MDA [14]. 4-HNE can be formed by multiple radical-dependent pathways involving hydroperoxides, alkoxyl radicals, epoxides, and fatty acyl crosslinking reactions [14]. AA is a phospholipid that makes up cell and organelle membranes, so the presence of MDA and 4-HNE signal both the general state of oxidative stress of the cell as well as the specific damage to lipid membranes of the cell [14]. Additionally, MDA and 4-HNE have adverse effects on the cell. MDA has been found to be mutagenic [14], and 4-HNE is highly reactive and toxic [22].
Lipid peroxidation products are widely used biomarkers of oxidative stress, and MDA and 4-HNE are the most commonly studied, with MDA levels being determined via its reaction with thiobarbituric acid (TBA) and 4-HNE by high-performance liquid chromatography (HPLC) [19]. In systemic disease, MDA and 4-HNE have been measured in many biological materials that include serum and urine as markers of oxidative stress [23]. Lipid peroxidation products have been used as biomarkers of oxidative stress in studies of inflammatory disorders such as atherosclerosis, asthma, rheumatoid arthritis [23], hypertension [24], cancer [25], and SLE [26], among others.
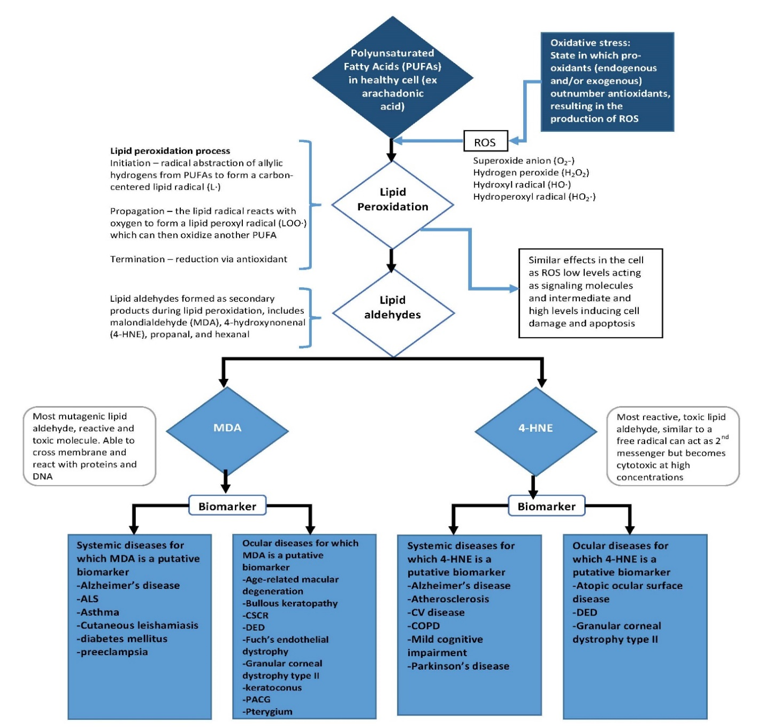
Figure 1:Overview of the pathway leading to the product of MDA and 4-HNE and their potential as biomarkers for systemic and ocular diseases.
MDA and 4-HNE: potential biomarkers for DED
The widespread findings that MDA and 4-HNE levels are significantly higher in the serum, tear samples, or cell samples of many inflammatory conditions, specifically including multiple ocular pathologies, indicate that MDA and 4-HNE levels could also be elevated in DED, a condition thought to involve oxidative stress. Tables 1 and 2 summarize all the studies to date looking at MDA and 4-HNE in DED and other ocular conditions. At this time, ex vivo studies have appeared to confirm this relationship, and limited in vivo studies have also suggested that MDA and 4-HNE could be elevated in patients with DED.
Ex vivo studies of MDA and 4-HNE
Ex vivo studies using Human Corneal Epithelial Cells (HCECs) have shown the relationship between a hyperosmolar environment (like that seen in DED) and a state of oxidative stress in the cell. The ex vivo nature of these studies has allowed for both the direct measurement of ROS as well as the measurement of downstream products of ROS, including the lipid peroxide products MDA and 4-HNE. One study established the correlation between an increasingly hyperosmolar environment, ROS production, and oxidative damage markers, including MDA and 4-HNE among others. In this study, HCECs from donor limbal explants were cultured in isosmolar (312 mOsM) or hyperosmotic (350, 400, 350 mOsM) media and ROS and markers of oxidative stress were measured using DCDFDA kit, RT-qPCR, immunofluorescence, and immunohistochemical staining [21]. Other similar studies have studied the effects of antioxidants, including L-carnitine [27] and pterostilbene [28], in reducing ROS and oxidative damage markers by culturing HCECs in hyperosmolar (450 mOsM) medium. The studies found that the presence of L-carnitine and pterostilbene caused a decrease in ROS and in MDA and 4-HNE. Furthermore, a study using HCECs has shown that the presence of 4-HNE decreases the viability of HCECs, with HCECs that were exposed to 4-HNE showing increased levels of ROS and decreased cellular expression of antioxidants and pro-inflammatory cytokines [29]. These ex vivo studies suggest that oxidative stress could play a key role in the pathogenesis of DED.
In vivo studies of MDA and 4-HNE in DED
Levels of MDA and 4-HNE in DED subjects have been evaluated in a few clinical trials, all be it with limited number of participants. One study looked at markers of oxidative stress in patients with Sjogren’s syndrome, an autoimmune condition that damages salivary and lacrimal glands leading to symptoms of severe dry mouth and dry eye. This study examined 31 eyes of 16 Sjogren’s syndrome patients and 15 eyes of 10 normal patients and found that a significantly higher percentage of conjunctival cells from DED patients stained positively for lipid oxidative stress markers 4-HNE as well as hexanoyl-lysine (HEL) when tested using immunohistochemical staining. In this study, 86.91 ± 7.25% of conjunctival cells from DED patient samples stained positive for 4-HNE compared to 26.37 ± 22.10% of conjunctival cells from healthy controls [30]. A later study examined patients with non-Sjogren syndrome DED to measure lipid oxidative stress markers MDA, 4-HNE, and HEL and their correlation to disease severity as measured by the tear film break-up time, Schirmer test value, tear clearance rate, keratoepitheliopathy scores, corneal sensitivity, conjunctival goblet cell density, and symptom score. This study examined 44 DED patients and 33 control patients for clinical signs and symptoms of DED and collected tears for immunohistochemical analysis. The study found a significant increase in the concentrations of MDA and 4-HNE in the tears of non-Sjogren DED patients compared to controls. The concentrations of MDA and 4-HNE found in the tears of DED patients was found to be 13.32 ± 4.03 pmol MDA/mg and 0.2 ± 0.03 μg 4-HNE/mL compared to 3.80 ± 4.03 pmol MDA/mg and 0.02 ± 0.01 μg 4-HNE/mL in healthy controls. This study also found that MDA and 4-HNE levels significantly correlated with tear film break-up time, Schirmer test value, tear clearance rate, keratoepitheliopathy scores, conjunctival goblet cell density, and symptom score; the only score that did not correlate with MDA and 4-HNE levels was corneal sensitivity [31].
Table 1: 4-HNE in DED and other ocular diseases
| Study description | Groups and interventions | n | mean ± SD | Conclusions |
|---|---|---|---|---|
| 4-HNE in DED | ||||
| In vivo studies | ||||
| To investigate the expression of lipid peroxidation markers in the tear film and ocular surface and their correlation with disease severity in patients with non-Sjögren dry eye disease (NSDED) [31] | Tears from healthy control patients | 33 | 0.02 ± 0.01 μg/mL* (p < 0.01) | • 4-HNE expression increases in the tear film and ocular surface of patients with DED
• 4-HNE levels correlate with various tear film and ocular surface parameters, may reflect the severity of DED |
| Tears from NSDED patients | 44 | 0.20 ± 0.03 μg/mL * (p < 0.01) | ||
| To evaluate the levels of lipid oxidative stress markers and inflammatory cells from tears and conjunctiva of patients with Sjögren syndrome (SS) and normal subjects [30] | Conjunctival cell sample from healthy control patients | 15 eyes (10 pts) | 26.37 ± 22.10%* (p < 0.0001)
(% of corneal epithelial cells staining positive for HNE) |
• A close relationship may exist between reactive oxygen species (ROS) production, lipid peroxidation related membrane damage, and inflammatory processes in dry eye |
| Conjunctival cell sample from SS patients | 31 eyes (16 pts) | 86.91 ± 7.25%* (p < 0.0001) | ||
| In vitro studies | ||||
| To explore the protective role and underlining mechanisms of pterostilbene (PS) in prevention of inflammatory injury in primary human corneal epithelial cells (HCECs) under hyperosmotic stress, an in vitro dry eye model [28]. | HCECs in 312 mOsM medium | 20.0%* (percentage of HCECs staining positive for HNE) | • PS protects human cornea from hyperosmolarity-induced inflammation and oxidative stress
• Suggests protective effects of PS on dry eye |
|
| HCECs in 450 mOsM medium | 84.0%* | |||
| HCECs in 450 mOsM medium with 10 uM PS added | 24.0%* | |||
| To determine if L-carnitine suppresses inflammatory responses in HCECs through suppression of ROS-induced oxidative damage in HCECs [27]. | HCECs in 312 mOsM medium | ~1 (ratio of 4-HNE: ß-actin) | • L-carnitine protects HCECs from oxidative stress by lessening the declines in antioxidant enzymes and suppressing ROS production
• L-carnitine reduces membrane lipid oxidative damage markers and mitochondrial DNA damage |
|
| HCECs in 450 mOsM medium | ~4.3 | |||
| HCECs in 450 mOsM medium with 20 uM L-Carnitine added | ~1.8 | |||
| To explore whether and how hyperosmolarity induces oxidative stress markers in primary HCECs [21] | HCECs in 312 mOsM medium | ~0.15* (ratio of 4-HNE: ß-actin) | • Hyperosmolarity induces oxidative stress in HCECs by stimulating ROS production and disrupting the balance of oxygenases and antioxidant enzymes
• Hyperosmolarity causes cell damage with increased oxidative markers in membrane lipid peroxidation and mitochondrial DNA damage |
|
| HCECs in 400 mOsM medium | ~0.35 | |||
| HCECs in 450 mOsM medium | ~0.5* | |||
| 4-HNE in other ocular diseases | ||||
| To investigate the influences of smartphone, use on ocular symptoms, status of the tear film, and oxidative stress indices in the tears and at the ocular surface [39] | Tear film at baseline | 50 | 10.08 ± 3.07 μg/mL | • Smartphone use could aggravate subjective symptom indices such as the OSDI, VAS, and CVS
• Smartphone use could induce tear film instability and oxidative stress indices in the tears and at the ocular surface. |
| Tear film after 1hr smartphone usage | 50 | 10.22 ± 3.03 μg/mL | ||
| Tear film after 4hr smartphone usage | 50 | 10.54 ± 3.32 μg/mL | ||
| Control tear film baseline | 30 | 9.76 ± 4.68 μg/mL | ||
| Control tear film after 1hr | 30 | 9.64 ± 3.36 μg/mL | ||
| Control tear film after 4hr | 30 | 9.68 ± 2.30 μg/mL | ||
| To clarify the presence of oxidative stress in patients with primary angle-closure glaucoma (PACG) and to investigate the relationship between oxidative stress and PACG [36] | Serum of healthy control patients | 50 | 14.16 ± 2.98 nmol/ml | • The serum 4-HNE concentrations were increased in PACG patients, but the differences with those of the healthy controls were not statistically significant. |
| Serum of patients with PACG | 50 | 15.25 ± 3.28 nmol/ml | ||
| To evaluate the ocular surface lipid oxidative stress status and inflammation in atopic keratoconjunctivitis (AKC) patients and normal subjects [33] | Conjunctival cell sample from healthy control patients | 18 eyes (9 pts) | 6.85 ± 14.13%* (p < 0.0001) | • The ocular surface disease in AKC was characterized by marked tear instability, ocular surface epithelial damage, increase in inflammatory infiltrates and presence of increased lipid oxidation. |
| Conjunctival cell sample from AKC patients | 28 eyes (14 pts) | 74.71 ± 17.15%* (p < 0.0001) | ||
Table 2: MDA in Ocular Disease
| Study description | Groups and interventions | n | mean ± SD | Conclusions |
|---|---|---|---|---|
| MDA in DED | ||||
| In vivo studies | ||||
| To investigate the expression of lipid peroxidation markers in the tear film and ocular surface and their correlation with disease severity in patients with non-Sjögren dry eye disease [31] | Tear film of healthy control patients | 33 | 3.80 ± 1.05 pmol/mg* (p < 0.01) | • Expression of MDA increases in the tear film and ocular surface of patients with dry eye
• The levels correlate with various tear film and ocular surface parameters and may reflect the severity of dry eye disease. |
| Tear film of patients with NSDED | 44 | 13.32 ± 4.03 pmol/mg* (p < 0.01) | ||
| In vitro studies | ||||
| To explore the protective role and underlining mechanisms of pterostilbene (PS) in prevention of inflammatory injury in primary HCECs under hyperosmotic stress, an in vitro dry eye model [28] | HCECs in 312 mOsM medium | 7.4%* (percentage of HCECs staining positive for MDA) | • PS protects human cornea from hyperosmolarity-induced inflammation and oxidative stress
• Suggests protective effects of PS on dry eye |
|
| HCECs in 450 mOsM medium | 81.4%* | |||
| HCECs in 450 mOsM medium with 10 uM PS | 29.6%* | |||
| To determine if L-carnitine suppresses inflammatory responses in HCECs through suppression of ROS-induced oxidative damage in HCECs [27] | HCECs in 312 mOsM medium | ~1* (ratio of MDA: ß-actin) | • L-carnitine protects HCECs from oxidative stress by lessening the declines in antioxidant enzymes and suppressing ROS production
• L-carnitine reduces membrane lipid oxidative damage markers and mitochondrial DNA damage |
|
| HCECs in 450 mOsM medium | ~3.8* | |||
| HCECs in 450 mOsM medium with 20 uM L-Carnitine added | ~1.5* | |||
| To explore whether and how hyperosmolarity induces oxidative stress markers in primary HCECs [21] | HCECs in 312 mOsM medium | ~0.45* (ratio of MDA: ß-actin) | • Hyperosmolarity induces oxidative stress in HCECs by stimulating ROS production and disrupting the balance of oxygenases and antioxidant enzymes
• Hyperosmolarity causes cell damage with increased oxidative markers in membrane lipid peroxidation and mitochondrial DNA damage |
|
| HCECs in 400 mOsM medium | ~0.95* | |||
| HCECs in 450 mOsM medium | ~1.3* | |||
| MDA in other ocular diseases | ||||
| To measure MDA in the tears of patients with central serous chorioretinopathy (CSCR) and investigate possible correlations with disease activity [38] | Healthy tears | 19 | 9914 ± 6126 nM | • Levels of MDA in tears correlate with RPE leakage in CSCR. |
| entire CSCR cohort tears | 31 | 7898 ± 6285 nM | ||
| acute CSCR tears | 8 | 12295 ± 8495 nM* | ||
| chronic CSCR tears | 23 | 6614 ± 4613 nM* | ||
| To investigate the serum changes of oxidative stress markers and the relationship between these factors and visual field (VF) progression in patients with primary angle closure glaucoma (PACG) [34] | Serum of healthy control patients | 89 | 5.57 ± 4.49umol/l* (p < 0.001) | • Higher serum MDA levels were associated with a higher risk of PACG
• Serum levels of MDA at baseline were associated with the progression of PACG as measured by the VF • Serum MDA could be useful marker for predicting the progression of patients with PACG • An imbalance in the oxidative stress system was involved in the onset and development of glaucoma • Oxidative stress might be a relevant target for both glaucoma prevention and therapy. |
| Serum of patients with PACG | 94 | 30.69 ± 35.99umol/l* (p < 0.001) | ||
| To determine the status of markers of oxidative stress in tears of patients with keratoconus (KC) following corneal crosslinking procedure [40] | Tears of healthy control patients | 20 | 95 ± 5 mmol/ml* | • Moderate KC patients MDA levels were significantly higher compared to mild KC patients
• MDA levels were significantly higher at 1 month following CXL • MDA levels were significantly lower at 3 months following CXL |
| KC patient tears baseline | 20 | 190 ± 20 mmol/ml* | ||
| KC patient tears 1-month post-crosslinking | 20 | 240 ± 25 mmol/ml* | ||
| KC patients tears 3 months post-crosslinking | 20 | 120 ± 15 mmol/ml* | ||
| To investigate the influences of smartphone, use on ocular symptoms, status of the tear film, and oxidative stress indices in the tears and at the ocular surface [39] | Tear film at baseline | 50 | 44.01 ± 6.03 pmol/mg | • Smartphone use could aggravate subjective symptom indices such as the OSDI, VAS, and CVS
• Smartphone use could induce tear film instability and oxidative stress indices in the tears and at the ocular surface. |
| Tear film after 1hr smartphone usage | 50 | 43.38 ± 4.71 pmol/mg | ||
| Tear film after 4hr smartphone usage | 50 | 45.14 ± 9.34 pmol/mg | ||
| Control tear film at baseline | 30 | 41.90 ± 11.22 pmol/mg | ||
| Control tear film after 1hr | 30 | 45.48 ± 14.62 pmol/mg | ||
| Control tear film after 4hr | 30 | 44.73 ± 9.45 pmol/mg | ||
| To compare serum levels of malondialdehyde (MDA) in patients with wet age-related macular degeneration (wAMD), patients with dry AMD (dAMD), and patients without AMD and to evaluate the efficacy of nutritional supplementation (S) for treating elevated serum MDA in patients with wAMD [41]. | wAMD serum baseline | 20 | 9.94 ± 1.53 pmol/ml* | • elevated serum MDA levels were directly associated with the area of CNV lesions in eyes with wAMD
• nutritional supplements appear to protect the eyes from systemic oxidative damage • MDA might be a valuable marker of oxidative stress |
| dAMD serum baseline | 20 | 9.30 ± 0.92 pmol/ml | ||
| control serum baseline | 24 | 9.04 ± 0.96 pmol/ml* | ||
| wAMD S+ group serum baseline | 10 | 10.34 ± 2.03 pmol/ml | ||
| wAMD S+ serum after nutritional supplement | 10 | 8.88 ±1.18 pmol/ml | ||
| wAMD S- group serum baseline | 10 | 9.54 ± 0.70 pmol/ml | ||
| wAMD S- serum after placebo supplement | 10 | 10.41 ± 1.36 pmol/ml | ||
| To compare MDA and total protein concentration in human tears in two different age groups: younger adults (18-30 years old) and elderly adults (65-85 years old) [42] | Tears of young adults (18-30 y/o) | 26 | 0.034 ± 0.021 mM/ul* (p = 0.0161) | • MDA concentration is increased in the tears of elderly people |
| Tears of old adults (65-85 y/o) | 40 | 0.051 ± 0.035 mM/ul* (p = 0.0161) | ||
| To clarify the presence of oxidative stress in patients with primary angle-closure glaucoma (PACG) and to investigate the relationship between oxidative stress and PACG [36]. | Serum of healthy control patients | 50 | 3.51 ± 0.84 nmol/ml* (p < 0.01) | • The concentration of MDA in PACG patients was significantly higher than those of the control subjects (P < 0.05) |
| Serum of patients with PACG | 50 | 4.35 ± 0.81 nmol/ml* (p < 0.01) | ||
MDA and 4-HNE in ocular surface disease or other eye diseases
The role of MDA or 4-HNE has also been explored in other ocular diseases besides DED. In corneal diseases, including keratoconus, bullous keratopathy, and Fuchs' endothelial dystrophy, MDA was found in cells of all diseased corneas and no healthy corneas [32]. MDA has been found to be elevated in pterygium tissue [7]. Both MDA and 4-HNE were measured in a study of granular corneal dystrophy type II (GCD II) and were found to be elevated in the corneal tissue of GCD II patients compared to controls [8]. In atopic ocular surface disease, 4-HNE was measured and was found to positively correlate with conjunctival inflammation [33]. MDA has been studied as a marker of glaucoma. One study found that serum levels of MDA were significantly higher in patients with primary angle-closure glaucoma compared to healthy subjects [34], and MDA has been found to be elevated in both serum and aqueous humour across multiple primary open angle glaucoma studies [35]. 4-HNE has not been as widely studied in glaucoma patients, but one study did look at 4-HNE in primary angle closure glaucoma patients and found that it was elevated in the serum of affected patients compared to normal controls, but not to a statistically significant level [36]. MDA has consistently been found to be elevated in the serum of patients with age-related macular degeneration [37]. A study of MDA in tear samples of patients with central serous chorioretinopathy (CSCR) found that MDA levels correlated to retinal pigment epithelium leakage and were significantly elevated in acute CSCR (12295 ± 8495 nM) compared to chronic CSCR patients (6614 ± 4613 nM) [38].
With many studies showing that MDA and/or 4-HNE have been elevated in states of disease or inflammation of the eye, MDA and 4-HNE have begun to be used as biomarker endpoints of experimental studies. MDA and 4-HNE levels in patient tear samples were used as biomarkers to assess the effects of smartphone usage on the ocular surface, and no significant difference was found between experimental (45.14 ± 9.34 pmol MDA/mg, 10.54 ± 3.32 μg 4-HNE/mL) and control subjects (44.73 ± 9.45 pmol MDA/mg, 9.68 ± 2.30 μg 4-HNE/mL) [39]. MDA was used to determine the effectiveness of a surgical crosslinking procedure used in patients with keratoconus, finding that MDA levels initially increased in the tears of keratoconus patients from baseline (190 ± 20 mmol/ml) to 1 month after the procedure (240 ± 25 mmol/ml) before decreasing within the range of healthy control patients after 3 months (120 ± 15 mmol/ml) [40]. A study has then been done to test the effect of nutritional supplements on wet age-related macular degeneration patients using serum MDA as a biomarker and found that MDA decreased in wAMD patients following nutritional supplementation [41].
The above studies of MDA and 4-HNE concentrations used a variety of methods. Some studies of wAMD or glaucoma measure levels of MDA or 4-HNE in the serum of patients, but the studies of ocular surface disease all measured MDA or 4-HNE using either conjunctival cells from small conjunctival biopsies or tears. The tears represent the best candidate for a potential biomarker because of the collection of tears is easily accessible and non-invasive. However, variability remains in the methods and units used in the study of tears. Daruich et al. used both the TBA method which is based on fluorescence detection of a TBA derivative after high-performance liquid chromatography (HPLC) separation and a strategy adapted from isolating MDA in urine samples in which 2-aminoacridone (2-AA) is used to form a selective detectable derivative of MDA. The TBA method is commonly used to detect MDA but has been criticized for being weakly specific and for harsh derivatization conditions that could lead to overestimation of MDA levels, and the 2-AA method has not been widely used and verified in tears [38]. Other studies, including Choi et al. and Matsuura et al. used commercially available ELISA kits for analysis of MDA and 4-HNE, but the results were reported using different units. While Choi et al. reported MDA concentrations in pmol MDA/mg of protein detected in tear samples, Matsuura et al. reported MDA concentration in pmol MDA/mL of tears [39 41]. Thus, results of these tear studies cannot be directly quantitatively compared due to different methodologies and units used.
Summary and future research
From the current literature, MDA and 4-HNE appear to be promising objective metrics for biomarkers for eye disease and analysis of tears allows for easy access if one can demonstrate specificity and repeatability of measurements related to ocular surface disease. In ex vivo tissue culture studies, MDA and 4-HNE increase in a hyperosmolar state that mimics that of DED, and the levels of MDA and 4-HNE positively correlate with the osmolarity of the medium. MDA and 4-HNE have widely been studied in a variety of clinical settings and have been also used as endpoint biomarkers in some experimental studies. Two studies have specifically looked at the potential for MDA and/or 4-HNE as biomarkers for DED, and both show that MDA and 4-HNE are elevated in DED- one in conjunctival cells, another in tears. Future research to better establish MDA and 4-HNE as biomarkers of DED are needed, such as establishing standardized processing methodology for analysis of tears. Current studies of MDA and 4-HNE use different methods and different units, so comparing levels of these markers cannot be done across studies. With a standardized methodology including units, normal baseline levels of MDA and 4-HNE could be established and confirmed across studies, and diagnostic levels of a diseased state could also be determined. Furthermore, the two published studies on these markers in DED had small samples sizes, giving the studies low power for generalization and broad usage. As this review demonstrates, MDA and 4-HNE can be elevated in many ocular diseases, so further clinical research is warranted to more clearly demonstrate their significance as related to patient signs and symptoms in DED and may offer a minimally objective metric to be used as a biomarker for DED.
Conclusions
These early studies indicate a promising potential for MDA and 4-HNE as biomarkers of DED in tears that indicate oxidative stress. The limited numbers of study participants, lack of standardized methodology of analysis and reporting of results, and their correlation with patient signs and symptoms indicate the need for more studies to establish MDA and 4-HNE as biomarkers of DED in tears.
References
- Kaludercic N, Deshwal S, Lisa FD. (2014) Reactive oxygen species and redox compartmentalization. Front Physiol. 5: 285. [PubMed.]
- Dogru M, Kojima T, Simsek C, Tsubota K. (2018) Potential Role of Oxidative Stress in Ocular Surface Inflammation and Dry Eye Disease. Invest Ophthalmol Vis Sci. 59(14): DES163-DES68. [Ref.]
- Baudouin C, Messmer EM, Aragona P, et al. (2016) Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 100(3): 300-306. [PubMed.]
- Seen S, Tong L. (2018) Dry eye disease and oxidative stress. Acta Ophthalmol. 96(4): e412-e420. [PubMed.]
- Pflugfelder SC, de Paiva CS. (2017) The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology. 124(11S): S4-S13. [PubMed.]
- Sacca SC, Cutolo CA, Ferrari D, Corazza P, Traverso CE. (2018) The Eye, Oxidative Damage and Polyunsaturated Fatty Acids. Nutrients. 10(6). [PubMed.]
- Balci M, Sahin S, Mutlu FM, Yagci R, Karanci P, et al. (2011) Investigation of oxidative stress in pterygium tissue. Mol Vis. 17: 443-447. [Ref.]
- Choi SI, Kim TI, Kim KS, et al. (2009) Decreased catalase expression and increased susceptibility to oxidative stress in primary cultured corneal fibroblastsfrom patients with granular corneal dystrophy type II. Am J Pathol. 175(1): 248-261. [Ref.]
- Jurkunas UV, Bitar MS, Funaki T, Azizi B. (2010) Evidence of oxidative stress in the pathogenesis of fuchs endothelial corneal dystrophy. Am J Pathol. 177(5): 2278-2289. [Ref.]
- (2007). The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf 2007. 5(2): 75-92. [PubMed.]
- Li S, Lu Z, Huang Y, et al. (2022) Anti-Oxidative and Anti-Inflammatory Micelles: Break the Dry Eye Vicious Cycle. Adv Sci (Weinh). e2200435. [PubMed.]
- Stapleton F, Alves M, Bunya VY, et al. (2017) TFOS DEWS II Epidemiology Report. Ocul Surf. 15(3): 334-365. [PubMed.]
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, et al. (1998) The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 17(6): 584-589. [PubMed.]
- Ayala A, Munoz MF, Arguelles S. (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014: 360438. [Ref.]
- Roy NS, Wei Y, Kuklinski E, Asbell PA. (2017) The Growing Need for Validated Biomarkers and Endpointsfor Dry Eye Clinical Research. Invest Ophthalmol Vis Sci. 58(6): BIO1-BIO19. [Ref.]
- Savini G, Prabhawasat P, Kojima T, Grueterich M, Espana E, et al. (2008) The challenge of dry eye diagnosis. Clin Ophthalmol. 2(1): 31-55. [PubMed.]
- (2016) BEST (Biomarkers, EndpointS, and other Tools) Resource. Silver Spring (MD). [Ref.]
- Frijhoff J, Winyard PG, Zarkovic N, et al. (2015) Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid Redox Signal. 23(14): 1144-1170. [PubMed.]
- Marrocco I, Altieri F, Peluso I. (2017) Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid Med Cell Longev. 2017: 6501046. [Ref.]
- Czerska M, Mikolajewska K, Zielinski M, Gromadzinska J, Wasowicz W. (2015) Today's oxidative stress markers. Med Pr. 66(3): 393-405. [PubMed.]
- Deng R, Hua X, Li J, et al. (2015) Oxidative stress markers induced by hyperosmolarity in primary human corneal epithelial cells. PLoS One. 10(5): e0126561. [Ref.]
- Schaur RJ, Siems W, Bresgen N. (2015) Eckl PM. 4-Hydroxy-nonenal-A Bioactive Lipid Peroxidation Product. Biomolecules. 5(4): 2247-2337. [PubMed.]
- Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. (2006) Biomarkers of oxidative damage in human disease. Clin Chem. 52(4): 601-623. [PubMed.]
- Verma MK, Jaiswal A, Sharma P, Kumar P, Singh AN. (2019) Oxidative stress and biomarker of TNF-alpha, MDA and FRAP in hypertension. J Med Life. 12(3): 253-259. [PubMed.]
- Lepara Z, Lepara O, Fajkic A, Rebic D, Alic J, et al. (2020) Serum malondialdehyde (MDA) level as a potential biomarker of cancer progression for patients with bladder cancer. Rom J Intern Med. 58(3): 146-152. [PubMed.]
- Wang G, Pierangeli SS, Papalardo E, Ansari GA, Khan MF. (2010) Markers of oxidative and nitrosative stress in systemic lupus erythematosus: correlation with disease activity. Arthritis Rheum. 62(7): 2064-2072. [Ref.]
- Hua X, Deng R, Li J, et al. (2015) Protective Effects of L-Carnitine Against Oxidative Injury by Hyperosmolarity in Human Corneal Epithelial Cells. Invest Ophthalmol Vis Sci. 56(9): 5503-5511. [Ref.]
- Li J, Ruzhi D, Hua X, et al. (2016) Blueberry Component Pterostilbene Protects Corneal Epithelial Cells from Inflammation via Anti-oxidative Pathway. Sci Rep. 6: 19408. [PubMed.]
- Liu H, Gambino F, Algenio CS, et al. (2020) Inflammation and oxidative stress induced by lipid peroxidation metabolite 4-hydroxynonenal in human corneal epithelial cells. Graefes Arch Clin Exp Ophthalmol. 258(8): 1717-1725. [PubMed.]
- Wakamatsu TH, Dogru M, Matsumoto Y, et al. (2013) Evaluation of lipid oxidative stress status in Sjogren syndrome patients. Invest Ophthalmol Vis Sci. 54(1): 201-210. [Ref.]
- Choi W, Lian C, Ying L, et al. (2016) Expression of Lipid Peroxidation Markers in the Tear Film and Ocular Surface of Patients with Non-Sjogren Syndrome: Potential Biomarkers for Dry Eye Disease. Curr Eye Res. 41(9): 1143-1149. [PubMed.]
- Buddi R, Lin B, Atilano SR, Zorapapel NC, Kenney MC, et al. (2002) Evidence of oxidative stress in human corneal diseases. J Histochem Cytochem. 50(3): 341-351. [PubMed.]
- Wakamatsu TH, Dogru M, Ayako I, et al. (2010) Evaluation of lipid oxidative stress status and inflammation in atopic ocular surface disease. Mol Vis. 16: 2465-2475. [PubMed.]
- Li S, Shao M, Li Y, et al. (2020) Relationship between Oxidative Stress Biomarkers and Visual Field Progression in Patients with Primary Angle Closure Glaucoma. Oxid Med Cell Longev. 2020: 2701539. [Ref.]
- Benoist d'Azy C, Pereira B, Chiambaretta F, Dutheil F. (2016) Oxidative and Anti-Oxidative Stress Markers in Chronic Glaucoma: A Systematic Review and Meta-Analysis. PLoS One. 11(12): e0166915. [Ref.]
- Chang D, Sha Q, Zhang X, et al. (2011) The evaluation of the oxidative stress parameters in patients with primary angle-closure glaucoma. PLoS One. 6(11): e27218. [PubMed.]
- Kersten E, Paun CC, Schellevis RL, et al. (2018) Systemic and ocular fluid compounds as potential biomarkers in age-related macular degeneration. Surv Ophthalmol. 63(1): 9-39. [PubMed.]
- Daruich A, Sauvain JJ, Matet A, et al. (2020) Levels of the oxidative stress biomarker malondialdehyde in tears of patients with central serous chorioretinopathy relate to disease activity. Mol Vis 2020. 26: 722-730 [PubMed.]
- Choi JH, Li Y, Kim SH, et al. (2018) The influences of smartphone use on the status of the tear film and ocular surface. PLoS One. 13(10): e0206541. [PubMed.]
- Balmus IM, Alexa AI, Ciuntu RE, et al. (2020) Oxidative stress markers dynamics in keratoconus patients' tears before and after corneal collagen crosslinking procedure. Exp Eye Res. 190: 107897. [PubMed.]
- Matsuura T, Takayama K, Kaneko H, et al. (2017) Nutritional Supplementation Inhibits the Increase in Serum Malondialdehyde in Patients with Wet Age-Related Macular Degeneration. Oxid Med Cell Longev. 2017: 9548767. [PubMed.]
- Benlloch-Navarro S, Franco I, Sanchez-Vallejo V, Silvestre D, Romero FJ, et al. (2013) Lipid peroxidation is increased in tears from the elderly. Exp Eye Res. 115: 199-205. [PubMed.]
