>Corresponding Author : Sumeyye Baser
>Article Type : Research Article
>Volume : 1 | Issue : 1
>Received Date : 08 Feb, 2023
>Accepted Date : 20 Feb, 2023
>Published Date : 27 Feb, 2023
>DOI : https://doi.org/10.54289/JPBT2300102
>Citation : Baser S, Kesli R and Ates MB. (2023) Evaluation of Microglia Activation Neuroinflammation and Neuronal Loss Presence in Experimentally Generated Rat Autism Model with Propionic Acid. J Psychol Behav Ther 1(1): 7-20.doi https://doi.org/10.54289/JPBT2300102
>Copyright : © 2023 Baser S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Research Article | Open Access
1Department of Medical Microbiology, Faculty of Medicine, University of Selcuk, Konya 42070, Turkey
2Department of Pathology, Faculty of Veterinary Medicine, University of Selcuk, Konya 42070,Turkey
*Corresponding author: Sumeyye Baser, Department of Medical Microbiology, Faculty of Medicine, University of Selcuk, Konya 42070, Turkey
Abstract
Objective: This study was conducted to determine the behavioral disorders and neuroinflammation that occur in the rat model of autism.
Materials and methods: A total of 25 rats, 20 of which were working and 5 were control group, were used in the study. The scores of the rats were recorded by performing a behavioral test before and after the application. A single dose of propionic acid (4 µl, 0.26 M solution) and (4 µl) saline were administered to the brain right ventricles of the rats in the working group and control group, respectively. Hippocampus tissue was obtained after the sacrification process on the 21st day of the rats and examined.
Results: Dystrophic changes with small, pycnotic and hyperchromatic nuclei of neurons in the cerebral cortex and hippocampal region were detected in histopathological studies. It was determined that the primitive cell layer thickness of the working group decreased significantly, and their density increased due to irregular chromatolysis in neurons and abnormal Nissl granule distribution. It was determined that the number of glial fibrillar acid protein positive cells increased significantly in the cerebral cortex and hippocampal region in propionic acid applied rats.
Conclusion: Propionic acid crosses the blood brain barrier. As a result, neuroinflammation, microglial activation and loss of myelin are observed in the brain.
Key words: Experimental autism model; Neuroinflammation; Microglia; Propionic acid; Rat
Abbreviations: ASD: Autism Spectrum Disorder, SCFA: Short Chain Fatty Acids, GABA: Gamma Amino Butyric Acid, HPA: Hypothalamus Pituiter Adrenal Axis: PPA: Propionic Acid, ICV: Intracerebroventricular, CG: Control Group, MWTT: Morris Water Tank Test, WG: Working Group, MWTT: Morris Water Tank Test, H&E: Hematoxylane Eozin, SPSS: Statistical Package for the Social Sciences
Introduction
Autism spectrum disorder (ASD) is an important lifelong health problem that affects one in 44 children [1]. ASD is generally characterized by behavioral disorders such as learning, speech, decline in social skills, successive meaningless repetitions and inability to provide eye contact [1]. The etiology of autism is not known for certain, there is no valid biological marker or biological test for the diagnosis of autism in children [2,3].
The intestinal microbiota, its nutrients consist mostly of carbohydrates. Non-digestible oligosaccharides, fermentation of carbohydrates leaking from digestion Bacteroides spp, Desulfovibrio spp, Bifidobacterium spp, Faecalibacterium spp. and Enterobacter spp. it is occured by synthesis of short chain fatty acids (SCFA’s) such as propionic acid, butyric acid and acetic acid thanks to bacteria such as [4]. Some microorganisms and lipopolisaccarides from impaired intestinal epithelial cells pass through microbiota products such as serotonin, gamma amino butyric acid (GABA), SCFA’s and activate the mucosal immune system, causing the release of inflammatory cytokines (such as IL-6, IL-1β, IFN-γ, TNF-α). It was determined that the communication between the intestinal microbiota and the brain can also occur through the vagal nerve by showing the decrease in immune response after vagotomy in the brain in the animal model of gastrointestinal tract infection [5]. Neuropeptides and hormones secreted by the arouse of enteric neurons (such as dopamine, serotonin, GABA, SCFA’s) affect brain function either through the vagal nerve or directly through the blood-brain barrier. Corticotropin releasing factor, which is secreted by vagal nerves or cytokines by activating the hypothalamus pituiter adrenal axis (HPA), increases intestinal permeability by increasing the release of intestinal cytokines [6,7]. Microbiota tryptophan can affect the pathways of serotonin and chinure, altering plasma levels of end products (kinurenic acid, quinolinic acid, serotonin) that can cross the blood-brain barrier and affect the brain [8]. Irregularities in the intestinal microbiota are directly or indirectly related to ASD symptoms using [4,9] type wealth [10-13] and changes in their abundance, immune system, certain metabolic pathways, abnormal behaviors [14] and severity of the disease [15].
Propionic acid (PPA) and other SCFA’s (acetic acid, butyric acid) affect central nervous system function, including neurotransmitter release and synthesis, inter-synapse communication, lipid metabolism, mitochondrial function, immune activation and gene expression [16,17]. In researches on experimental animals, the potential effect of PPA with ASD has been identified. PPA, which is administered as intracerebroventricular (ICV), has been observed to impair social behavior and interaction [16,18-20]. Anxiety, social interaction disorders, and glial cell activation in the brain were detected by systemic application of PPA to rats [21]. Neuroinflamation, activity and proliferation of glial cells (astrocytes and microglia) occur as a result of injury, infection or disease. Microglia and astrocytes undergo morphological changes and secrete many pro-inflammatory agents in response to these pathologies [22,23]. These agents consist primarily of cytokines and chemokins that coordinate immunity under physiological conditions and regulate neuronal function and connectivity. Therefore, increased microglia or astrocyte activity leads to abnormal immunity. It then affects neuronal signaling and cognitive function [24].
In this study, it was aimed to evaluate the presence of microglia activation, neuroinflammation and neuronal loss by creating an experimentally model of autism in rats.
Materials and Methods
1. Ethical approval
Ethical approval maintained from Selcuk University Experimental Research and Application Center Experimental Animals Local Ethics Committee (25.10.2019/2019-47).
2. Supply and Maintenance of Rats
The research was conducted using 8-12 weeks of Wistar albino with an average weight of 250-300 grams using a total of 25 male rats at Selçuk University Experimental Research and Application Center (Konya, Turkey). During the experiment, rats were kept in rooms with an ambient temperature of 21° C and humidity of 55-60%, 12 hours of light (08:00-20:00 hours), 12 hours of darkness and fed with ad libitum rat feed and tap water.
3. Design of Study Groups
Before starting the experiment, the animals was divided into 2 groups, as follows, equal to their weight.
1- Control Group (CG n = 5): For thirty days, the name libitum was fed with rat feed, morris water tank test (MWTT) was applied in the first 9 days and 11.-20 days. ICV serum physiological (SF) injection was performed on the 10th day.
2- Working Group (WG n = 20): MWTT was applied in the first 9 days and 11.-20 days. ICV PPA injection was performed on the 10th day.
1 of the 20 rats in the working group was removed from the study due to anesthetic stress or stress caused by environmental conditions.
4. Intracerebroventricular Injection
The right ventricular region of rats was detected by topographic anatomical location method with stereotaxy device (World Precision Instruments, Inc, Model 502600, United Kingdom). Anesthesia was applied with 10 mg/kg rompun (Xylazinbio 2%, Bioveta, Czech Republic) and 70 mg/kg of ketamine (Ketasol 10%, Richter Pharma Ag, Austria) intraperitoneal so that they do not suffer pain and pain immediately before application. After the animals physiological responses (such as finger pinching) were followed and under the influence of anesthesia, their heads were shaved and ready for the operation. The animals were then placed parallel to the ground, with their ears and mouth fixed to the stereotaxic device. After the scalp was opened sagitally with the help of a scalp, the bregma point was determined and a hole was drilled in the ventricle according to the stereotaxsi coordinates. Ventricular reference point anterior/posterior -1.4 mm over bregma; medial/lateral 1.8 mm; determined by going dorsal/ventral -3.0 mm. The skull bone was pierced, a single dose (4 µl) of saline was given to the control group and a single dose of (4 µl, 0.26 M) PPA to the working group. After the injection completed, the animal was removed from the device and the incision area was closed with stitches.
5. Morris Water Tank Test (MWTT)
A tank filled with water (90 cm diameter, 40 cm height) and a platform with a height of 25 cm, a diameter of 10 cm and a distance of 5 cm from the tank edge are prepared in the north-east direction. The tank; it was divided into four dials as a dream: north, south, west, east. The center of each dial was marked with indicators of various colors and colors and was fixed around the tank so that animals can see it. Rats in the control group and working group were dropped into the water from a dial and their reach to the platform was recorded.
6. Euthanasia, Brain Tissue Dissection and Macroscopic Examination
Anesthesia was applied by injecting 10 mg / kg rompun (Xylazinbio 2% Biovet, Czech Republic) and 70 mg / kg ketamine (Ketasol 10%, Richter Pharma AG, Austria) intraperitoneally to all the rats, and than euthanized under anesthesia. After cervical dislocation was performed, the calvarium was removed and the brain was removed. Brain, cerebellum and brain stem were divided into two with said macroscopic examinations after a sagittal section through these tissues. The right hemisphere, cerebellum and brain stem were placed in 10% buffered formaldehyde solution for histopathological and immunohistochemical examinations.
7. Histopathological Examination
7.1. Hematoxylane Eozin (H&E) Painting
After the tissues were washed for 24 hours, histopathological follow-up was performed on the tissue tracking device (Leica TP 1020). Paraffin-blocked tissues were cut with a microtome (Leica RM 2125RT) at 5µ thickness and kept in an oven at 37°C for 24 hours. All sections were repeated three (3 x 5 min) was deparaffinized in xylene series of graded alcohols (100-70%) was passed. Then, sections [25] stained with Hematoxylin-Eosin (H&E) were examined under a 5-attach binocular head light microscope (Olympus BX51, Tokyo, Japan). Photographs of histopathological findings (Olympus, EP50) were taken.
7.2. Nissl Painting Method
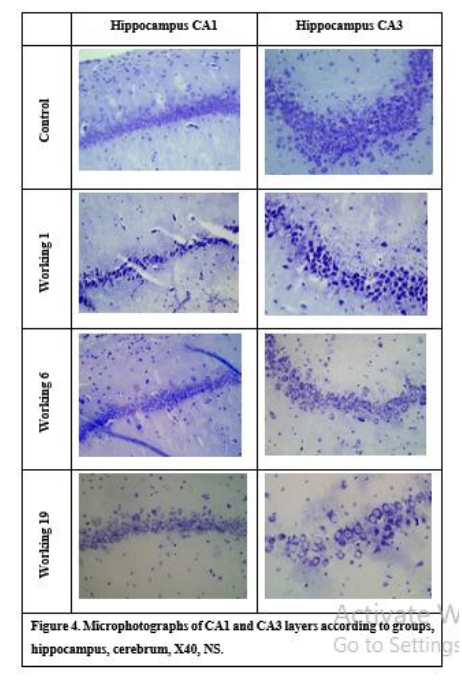
Coronal sections transferred to 5µ thick positively charged slides were first passed through xylol (3 x 5 min) and alcohol series (100-70%). Then, it was stained with 0.1% Cresyl violet solution for 5 minutes at 37 °C. After washing with distilled water, it was decolorized in 95% ethanol for 5 minutes. After dehydration and polishing with xylol (2x5 min), it was covered with mount and examined under a light microscope (Olympus BX51, Tokyo, Japan). Cerebral cortex thickness was found by averaging measurements made with a 4x objective (EPview 1.2, Olympus Soft Imaging Solutions GmbH, Germany) (Figure 1). Pyramidal layer thickness of CA1 and CA3 parts of hippocampus were determined by EPview 1.2, cell counts and cell staining intensity were determined by Imaje J (1.54d version, National Institutes of Health, USA) software.
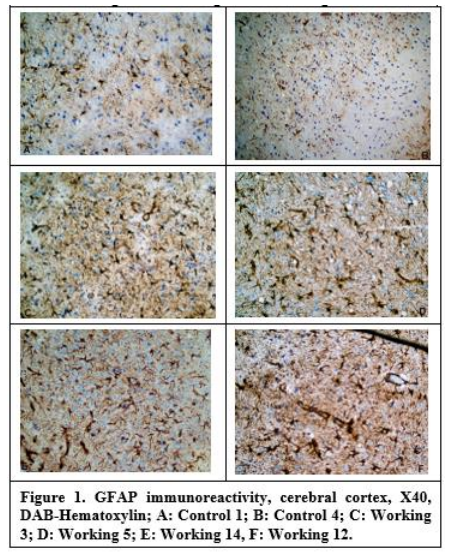
8. Immunohistochemical Examination
Staining, sagittal sections covering the brain, cerebellum and brainstem were stained on the Leica Bondmax stainer according to the Bond™ Polymer Refine Detection (Leica DS9800) kit protocol. First, all tissues were dewaxed with heat and dewax solution (Bond™, Leica AR9222) and serially rehydrated in increasing grades of alcohols (100-70%) (Sigma). According to the protocol, special washing solution (Bond™, Leica, AR9590) and/or distilled water was washed 3 times after each chemical or marker. To all slides; heat induced antigen retrieval (HIER 1, Citrate Buffer Ph:6.0, Leica, AR9961) was applied. Peroxidase and protein block application was performed to prevent non-specific staining. In follow primary antibodies (2; anti- CD68 antibody (Abcam, ab32503, USA) and anti-GFAP antibody (Abcam, ab59348 USA)) at room temperature in the primary post after incubation times ranging and polymer application was made. All sections, chromogen (DAB) was allowed to react for 3 minutes and washed with distilled water. It was then dehydrated by counterstaining with Mayer's Hematoxylin and sealed with entellan. The stained tissue under a light microscope (Olympus BX51, Tokyo, Japan) were photographed and examined the necessary (EP50 Olympus, Tokyo, Japan). In the evaluation of the staining, the evaluation system based on the number of stained cells in the examined areas was taken as basis. Accordingly, scores were determined according to the average of the number of positively stained cells in 5 different areas determined at 40 magnification in the hippocampus region in each section.
9. Statistical analyses
9.1. Behavioral Test Statistical Analyses
SPPS 25 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.) statistical package program was used to evaluate the data. Parameter results are expressed as mean ± standard deviation and minimum-maximum.
Before-After and day parameters were compared between groups by repeated measures of variance (ANOVA), (post hoc Bonferroni) test. P values less than 0.05 were considered statistically significant.
9.2. Histopathology Statistical Analyses
All statistical analyses were performed with statistical package for the social sciences (SPSS for Windows® version 25.0). In order to determine whether the data showed a normal distribution, Kolmogrov – Smirnov and homogeneity of variances were made with Levene test. Then, all data were analyzed with independent sample t-test and the results were obtained as mean ± standard errors (X̅ ± Sx̅). P values less than 0.05 were considered statistically significant
Results
1. Morris Water Maze Test (MST) Results There was a statistically significant difference between the time Before-After averages (p = 0.001<0.05, F = 22.978). There was a statistically significant difference between the day averages (p = 0.001<0.05, F = 28.728).There was a statistically significant difference between group averages in the Before-After*Day interaction (p = 0.001<0.05, F = 69.820). When the before and after groups were examined on a daily basis, the difference with all days was statistically significant (p = 0.001<0.05) (Table 1). It was determined that there was a gradual decrease after the 1st day and the lowest average duration of the 8th day while the 1st day was the highest average in the group. Then, on the contrary, it was observed that the lowest average period increased gradually from day 1 today.
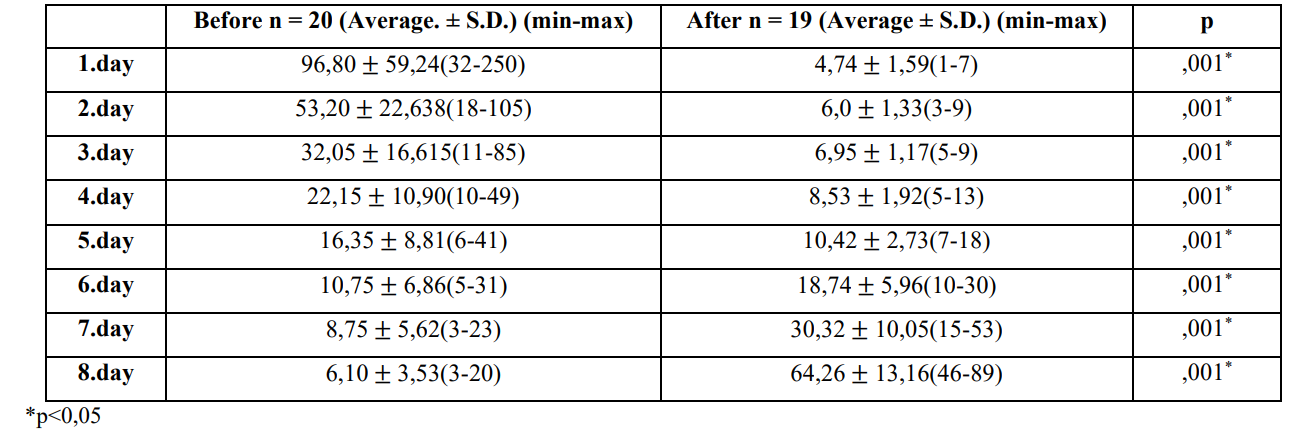
Table 1: Average platform finding times before and after study group PPA injection.
2. Histopathological Results
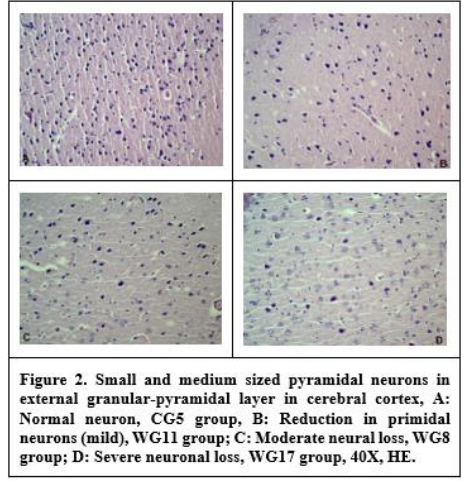
In the H&E staining, hyperemia in the meninges of all rats in the working group, bleeding and inflammatory reaction were observed in some. In addition, edema, hemorrage, degeneration, and necrosis in neurons, neuronophages, and gliosis were found. In the cerebral cortex section of the control group rats, a slight increase in neuropil tissue was determined without an increase in the number of neurons. There was increase in the neuropil tissue without an increase in the number of neurons in the cerebral cortex of the control group rats. It was observed that especially pyramidal neurons in this region showed an irregular orientation. Hyperemia, bleeding and inflammatory reactions were detected in the ependymal and subependymal regions of the ventricles. Degenerated neurons in the cerebral cortex and hippocampal region (especially the CA1 and CA3 regions in the primidal layer) were found to show dystrophic changes with small, pycnotic and hyperchromatic nuclei. In addition, it was determined that their density increased due to irregular chromatolysis and abnormal Nissl granule distribution in these neurons. Dilatation and hyperemia in the vessels were also observed. It was determined that the number of neurons especially in the external granular and pyramidal layers of the cerebral cortex was decreased (Figure 2).
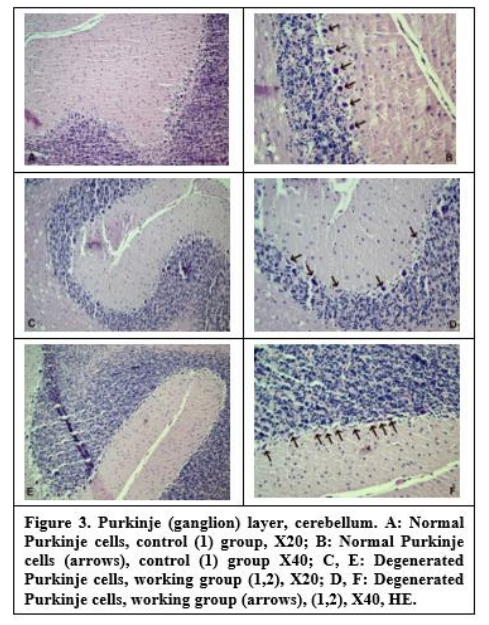
In the cerebellums of the working group rats, Purkinje cells had irregular borders, eosinophilic cytoplasm, and unspecified picnotic small nuclei. Perineural spaces were seen around Purkinje cells. While empty areas are detected in the places of Purkinje cells; granular layer was unaffected (Figure 3).
In the Nissl staining examination, the cerebral cortex thickness was 480.24-543.27 micrometers (𝜇m) in the control group and 417.49-621.82 𝜇m in the working group. Although thickening was observed in the working group, this was not statistically significant. Hippocampus CA1 region thickness was 23.94-26.1 μm in the control group and 11.82-28.89 μm in the working group. It was determined that the CA1 pyramidal layer thickness of the working group decreased significantly (P<0.01). The number of cells in this region was found to be significantly lower in the working group (P<0.01). The pyramidal cell layer thickness of the hippocampus CA3 region was 48.13-55.58 µm in the control group and 17.35-55.28 µm in the working group. A significant decrease in CA3 pyramidal cell layer thickness was observed in the working group (P<0.01) (Figure 4). According to each case staining intensity values of pyrimidal cells in hippocampus CA1 and CA3 regions; minimum 125.45, maximum 158.33 for CA1 region; for the CA3 region, it was found to be a minimum of 126.14 and a maximum of 164.13. Hippocampus CA1 pyramidal layer density was significantly increased in the working group (P<0.05). It was statistically determined that the cells in the hippocampus CA3 pyramidal layer were stained more intensively in the working group (P<0.05).
3. Immunohistochemical results
No immunoreactivity that could be evaluated in neuropil tissues of rats was found in staining for CD68. As a result of staining for GFAP, the averages of positive cells counted in 5 different areas were determined at 40X magnification. It was determined that the number of GFAP positive cells increased statistically in both the cerebral cortex and hippocampal regions of rats treated with PPA (P<0.01) (Figure 5-1).

Discussion
In recent studies, experimental ASD models have been created with valproic acid, lipopolysaccharide, PPA and stool transfers targeting intestinal microbiota due to the difficulty of working on humans [26-33]. In order to understand whether the model is formed in these models, first behavioral tests and then blood, tissue, organ, etc. to be studied. investigations are underway.
Various tests such as Morris water test, three-circle sociability test and direct sociability test are widely used in experimental animal models to test hippocampal-dependent learning and memory in the study of neurodevelopmental disorders. Behavioral changes of animals before and after the application are determined in order to understand whether the model is formed in ASD models [20,33]. In a study conducted in Canada, 20 male rats were given PPA to evaluate cognitive and motoric activity in the experimental autism model created with PPA and it was determined that they exhibited negative behaviors compared to the controls [34]. In another study, as a result of behavioral tests (T-maze task, object orientation, social behavior test) performed in the experimental autism model created with PPA given as ICV, it was determined that rats given PPA exhibited negative behaviors compared to controls [20]. When the MWTT results were evaluated, it was determined that learning skills and social behaviors decreased in the working group compared to the control group. When the research on experimental ASD modeling and the behavioral test results of our study are evaluated together, ICV PPA injection creates ASD-like behavioral disorders in animals and causes negative results in the cognitive, social and behavior of the models.
In addition to behavioral disorders, the increase in brain volume in ASD is mainly due to the increase in white and gray matter in the cerebral cortex, cerebellum and limbic structures of the brain. In addition, there is an increase in volume in the frontal, temporal and parietal lobes of the brain. In the frontal lobe, where the highest volume increase was detected, especially in the dorsolateral prefrontal cortex and medial frontal cortex, volume increase was detected. Studies have shown changes in the structure of cells in the cortex of the anterior cingulate gyrus, hippocampus, amygdala, subiculum, septal nuclei and mammillary body [18-20,34-40].
In the current study, degenerated neurons in the cerebral cortex and hippocampal region (especially in the CA1 and CA3 region primidal layer) showed dystrophic changes with small, picnotic and hyperchromatic nuclei. In addition, irregular chromatolysis, increased densities due to abnormal Nissl granule distribution, dilatation and hyperemia in vessels were observed in these neurons. It was determined that the number of neurons in the external granular and pyramidal layers of the cerebral cortex decreased. Neuronal losses in the cerebral cortex and hippocampus are thought to have occurred in parallel with the data and other studies obtained as a result of damage to the brain with PPA.
Decreased purkinje cell density in the cerebellum, abnormalities in the dentate nucleus, tendency of olivari cells towards the periphery of the convolution, changes in the number and size of inferior olivarial and cerebellar nucleus neurons were detected [18-20,34,41,42].
In this study, it was observed that purkinje cells had irregular borders, eosinophilic cytoplasm and indistinct pycnotic small nuclei in the cerebellum of the working group rats. Perineural empty areas were seen around purkinje cells. In line with our neuropathological studies and study data, there is a decrease in the number of purkinje and perineuronal empty areas in the cerebellum.
In a study in which an experimental ASD model was created by ICV injection of PPA into the brain, a significant increase in GFAP immunoreactivity was detected in the brain tissue taken. It was determined that this increase was mostly in the hippocampus and in white matter. Likely microglia activation was observed in the hippocampus and white matter for CD68. [18] In another study, an increase in the number of astrocytes was observed in all regions of the brain. No changes were detected in hippocampal pyramidal cells [19].
GFAP immunoreactivity increased in the CA3 region and the CA1/CA2 region of the hippocampus and the external capsule as a result of the analysis of brain tissue taken from rats given PPA to evaluate cognitive and motoric activity. However, microglia activation was detected for CD68 in CA3, dentate gyrus and white matter. In line with the staining, more intense staining was observed in the relevant regions, especially in the white matter and perivascular regions [34]. It was determined that GFAP and CD68 immunoreactivity, which were examined in the hippocampus and white matter, were more increased in adult rats in the brain histopathological examinations of rats in which experimental autism model was developed with PPA on adolescent and adult rats. No loss of hippocampal pyramidal cells was detected in adolescent rats [20].
When the immunohistochemical stainings for CD68 and GFAP were evaluated in our study; In immunohistochemical stainings performed for CD68, CD68 immunoreactivity of both control and working group rats was found in the meninges, ventricular ependymal cells, vascular endothelial cells in all cases, and microglial cells in a very few cases.
It has been observed that this reaction spreads from the meninges to the neuropil tissue from time to time. It was determined that the number of GFAP positive cells increased in both the cerebral cortex and hippocampal region in PPA administered rats. As a result of the comparing hippocampus CA1 pyramidal layer densities by group, it was determined that the staining densitiy in the working group increased compared to the control group. Similarly, cells in the CA3 pyramidal layer of the study hippocampus were stained more intensively than in the control group. It was determined that the pyramidal layer thickness of the hippocampus CA1 and CA3 decreased significantly in the working group (P<0.01). Similarly, the number of cells in this region was found to be significantly lower in the working group compared to the control group. In addition, as a result of the comparison of the hippocampus CA1 and CA3 pyramidal layer densities according to the groups, it was determined that the staining intensity in the working group increased significantly compared to the control group (P<0.01).
Conclusions
As a result, ASD represents as a big puzzle picture on a wall. The picture on the wall should be environed fully and obsorved correctly. Otherwise, in the absence of any of the parts that make up the big picture, it is not possible to put that part in the right place to complete the integrity of the picture. It is difficult to study the etiopathology and appropriate treatment methods of ASD on humans. Appropriate treatment methods should be developed to explain the etiopathology. Experimental ASD animal models can make contribution to shed light on the mysterious aspects of the autism such as neuroimmunpathologis seen in the brain. Explanation of the pathogenesis of the disorder also may lead to maintain clues and new discoveries for treatment approaches. Etiological causes and changes in tissues and organs such as brain tissue and intestinal microbiota should be contributed to the determination and enlightenment. In this study, we hope to contribute to the etiopathology of ASD by explaining neuroinflammation, microglia activation and neuronal losses that occur with the experimental ASD model created with PPA induction.
Author Contributions: Conceptualization, S.B, R.K and M.B.A; methodology, S.B, R.K and M.B.A; validation, S.B, R.K and M.B.A; formal analysis, S.B, R.K and M.B.A; investigation, S.B, R.K and M.B.A; resources, S.B, R.K and M.B.A; data curation S.B, R.K and M.B.A; writing-original draft preparation, S.B, R.K and M.B.A; writing-review and editing, S.B, R.K and M.B.A; visualization S.B, R.K and M.B.A; supervision S.B, R.K and M.B.A; project administration, S.B, R.K and M.B.A
Funding: This study was supported by Selcuk University Coordinator ship of Research Projects (Project No: 20202006).
Limitations: Not applicable.
Institutional Review Board Statement: Ethical approval maintained from Selcuk University Experimental Research and Application Center Experimental Animals Local Ethics Committee (25.10.2019/2019-47).
Informed Consent Statement: Not applicable.
Data Availability Statement: There's no data used in the process.
Acknowledgements: This study was supported by Selcuk University Coordinator ship of Research Projects (Project No: 20202006). This study was presented as an oral presentation at the Microbiome Therapies Congress with International Participation between 17-20 March 2022 in Antalya / Turkey. Thanks to the Research team for their scientific contributions and insights in the preparation of the study with the principles of high ethics, honesty, and openness.
Conflicts of Interest: The authors declare no conflict of interest.
References
- Maenner MJ, Shaw KA, Bakian AV, BiderDA, Durkin MS, et al. (2021) Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites United States, 2018. MMWR Surveill Summ. 70(11): 1-16. [PubMed.]
- Abrahams BS, Geschwind DH. (2008) Advances in autism genetics: on the threshold of a new neurobiology Nat Rev Genet. 9(5): 341-355. [Ref.]
- Ameis SH, Szatmari P. (2012) Imaging-genetics in autism spectrum disorder: advances translational impact and future directions Front Psychiatry. 346. [PubMed.]
- Borre YE, O'Keeffe GW, Clarke G, Stanton C, Dinan TG, et al. (2014) Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 20(9): 509-518. [PubMed.]
- Wang X, Wang BR, Zhang XJ, Xu Z, Ding YQ, et al. (2002) Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J Gastroenterol. 8(3): 540-545. [PubMed.]
- Yarandi SS, Peterson DA, Treisman GJ, Moran TH, Pasricha PJ. (2016) Modulatory Effects of Gut Microbiota on the Central Nervous System: How Gut Could Play a Role in Neuropsychiatric Health and Diseases. J Neurogastroenterol Motil. 22(2): 201-212. [PubMed.]
- Li Q, Han Y, Dy ABC, Hagerman RJ. (2017) The Gut Microbiota and Autism Spectrum Disorders Front Cell Neurosci. 11120. [Ref.]
- O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. (2015) Serotonin tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 27732-27748. [PubMed.]
- de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, et al. (2010) Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 51(4): 418-424. [PubMed.]
- Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. (2011) Gastrointestinal flora and gastrointestinal status in children with autism-comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 11: 22. [PubMed.]
- De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, et al. (2013) Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 8(10): e76993. [Ref.]
- Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, et al. (2010) Pyrosequencing study of fecal microflora of autistic and control children Anaerobe. 16(4): 444-453. [PubMed.]
- Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, et al. (2002) Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 35(1): 6-16. [PubMed.]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, et al. (2013) Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders Cell. 155(7): 1451-1463. [PubMed.]
- De Angelis M, Francavilla R, Piccolo M, De Giacomo A, Gobbetti M. (2015) Autism spectrum disorders and intestinal microbiota Gut Microbes. 6(3): 207-213. [PubMed.]
- Macfabe DF. (2012) Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Health Dis. 23. [PubMed.]
- Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. (2016) From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites Cell. 165(6): 1332-1345 [PubMed.]
- MacFabe DF, Cain DP, Rodriguez-Capote K, Franklin AE, Hoffman JE, et al. (2007) Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders Behav Brain Res. 176(1): 149-169. [PubMed.]
- Shultz SR, MacFabe DF, Ossenkopp KP, Scratch S, Whelan J, et al. (2008) Intracerebroventricular injection of propionic acid an enteric bacterial metabolic end-product impairs social behavior in the rat: implications for an animal model of autism Neuropharmacology. 54(6): 901-911. [PubMed.]
- MacFabe DF, Cain NE, Boon F, Ossenkopp KP. (2011) Cain DP Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior social behavior cognition and neuroinflammation in adolescent rats: Relevance to autism spectrum disorder Behav Brain Res. 217(1): 47-54. [PubMed.]
- Shams S, Foley KA, Kavaliers M, MacFabe DF, Ossenkopp KP. (2019) Systemic treatment with the enteric bacterial metabolic product propionic acid results in reduction of social behavior in juvenile rats: Contribution to a rodent model of autism spectrum disorder Dev Psychobiol. 61(5): 688-699. [PubMed.]
- Hanisch UK, Kettenmann H. (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain Nat Neurosci. 10(11): 1387-1394. [PubMed.]
- Kierdorf K, Prinz M. (2013) Factors regulating microglia activation Front Cell Neurosci. 744. [Ref.]
- Matta SM, Hill-Yardin EL, Crack PJ. (2019) The influence of neuroinflammation in Autism Spectrum Disorder Brain Behav Immun. 7975-7990. [PubMed.]
- Luna LG. (1968) Manual of histologic staining methods of the Armed Forces Institute of Pathology [Ref.]
- El-Ansary AK, Al-Daihan SK, El-Gezeery AR. (2011) On the protective effect of omega-3 against propionic acid-induced neurotoxicity in rat pups Lipids Health Dis. 10142. [PubMed.]
- Foley KA, MacFabe DF, Vaz A, Ossenkopp KP, Kavaliers M. (2014) Sexually dimorphic effects of prenatal exposure to propionic acid and lipopolysaccharide on social behavior in neonatal adolescent and adult rats: implications for autism spectrum disorders Int J Dev Neurosci. 3968-3978. [PubMed.]
- Schneider C. (2005) Chemistry and biology of vitamin E Mol Nutr Food Res. 49(1): 7-30. [PubMed.]
- Bilbo SD, Block CL, Bolton JL, Hanamsagar R, Tran PK. (2018) Beyond infection - Maternal immune activation by environmental factors microglial development and relevance for autism spectrum disorders Exp Neurol. 299(A): 241-251. [PubMed.]
- Choi J, Lee S, Won J, Jin Y, Hong Y, et al. (2018) Pathophysiological and neurobehavioral characteristics of a propionic acid-mediated autism like rat model PLoS One. 13(2): e0192925. [PubMed.]
- Kirsten TB, Bernardi MM. (2017) Prenatal lipopolysaccharide induces hypothalamic dopaminergic hypoactivity and autistic-like behaviors: Repetitive self-grooming and stereotypies. Behav Brain Res. 331: 25-29. [PubMed.]
- Foley KA, Ossenkopp KP, Kavaliers M, Macfabe DF. (2014) Pre- and neonatal exposure to lipopolysaccharide or the enteric metabolite propionic acid alters development and behavior in adolescent rats in a sexually dimorphic manner. PLoS One. 9(1): e87072. [PubMed.]
- Sharon G, Cruz NJ, Kang DW, Gandal MJ, Wang B, et al. (2019) Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice Cell. 177(6): 1600-18 e17. [PubMed.]
- Shultz SR, Macfabe DF, Martin S, Jackson J, Taylor R, et al. (2009) Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long-Evans rat: further development of a rodent model of autism Behav Brain Res. 200(1): 33-41. [PubMed.]
- Carper RA, Moses P, Tigue ZD, Courchesne E. (2002) Cerebral lobes in autism: early hyperplasia and abnormal age effects Neuroimage. 16(4): 1038-1051. [PubMed.]
- Carper RA, Courchesne E. (2000) Inverse correlation between frontal lobe and cerebellum sizes in children with autism Brain. 123: 836-844. [PubMed.]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, et al. (2002) Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 59(2): 184-192. [PubMed.]
- Carper RA, Courchesne E. (2005) Localized enlargement of the frontal cortex in early autism Biol Psychiatry 57(2): 126-133. [PubMed.]
- Kemper TL, Bauman M. (1998) Neuropathology of infantile autism. J Neuropathol Exp Neurol. 57(7): 645-652. [PubMed.]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia Nature. 541(7638): 481-487. [Ref.]
- Bauman M, Kemper TL. (1985) Histoanatomic observations of the brain in early infantile autism Neurology 35(6): 866-874. [PubMed.]
- Kemper TL, Bauman ML. (1993) The contribution of neuropathologic studies to the understanding of autism Neurol Clin. 11(1): 175-187. [PubMed.]
