>Corresponding Author : Nerenxa Ajasllari
>Article Type : Case Report
>Volume : 3 | Issue : 3
>Received Date : 13 March, 2023
>Accepted Date : 23 March, 2023
>Published Date : 28 March, 2023
>DOI : https://doi.org/10.54289/JCRMH2300112
>Citation : Ajasllari N, Zoto A, Rapushi E, Todhe C and Ajasllari A. (2023) A Non-Hodgkin’s Lymphoma Case in a Patient with Sjogren Syndrome and Covid-19. J Case Rep Med Hist 3(3): doi https://doi.org/10.54289/JCRMH2300112
>Copyright : © 2023 Ajasllari N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access
1University Hospital Center “Mother Teresa”, Department of Rheumatology, Tirana, Albania
2Specialties Polyclinic Nr. 2, Department of Nephrology, Tirana, Albania
*Corresponding author: Nerenxa Ajasllari, University Hospital Center “Mother Teresa”, Department of Rheumatology, Tirana, Albania
Abstract
Background: Sjogren syndrome (SS) is a chronic autoimmune disorder of the exocrine glands, accompanied by lymphocyte infiltration of these glands, that manifests clinically with dryness of the eyes (lacrimal glands involvement) and of the mouth (salivary glands involvement). SS can develop extraglandular manifestations and complications such as hematologic malignant transformation. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly transmissible coronavirus discovered in 2019, causing a world pandemic by developing an acute respiratory disease ‘coronavirus disease 2019’ (COVID-19). We are presenting a case with pSS, complicated with Non Hodgkin Lymphoma (NHL), after infection with SARS-CoV-2.
Case presentation: The patient is a 52 year old female, who presented in the clinic complaining of dryness of the eyes and mouth, swollen nodule in the neck region. According to European League Against Rheumatism (EULAR) and American College of Rheumatology (ACR) criteria, the patient is diagnosed with SS and starts treatment with Plaquenil 200mg/day, Medrol 4mg/day, Calcium, D3 vitamin, Proton-pump inhibitor (PPI). After infection with SARS-CoV-2, the patients has aggravation of her symptoms related to SS and in a time frame of 8 months she manifests weakness, tiredness and weight loss and generalized lymphadenopathy. Biopsy is performed confirming the diagnosis of Non Hodgkin Lymphoma.
Conclusions: This clinical case presentation tries to explain a possible relation between COVID-19 and hematologic malignant transformation in predisposed patients, like patients with pSS.
Keywords: Primary Sjorgen Syndrome; COVID-19; Non Hodgkin Lymphoma; Malignant Hematologic Complication
Abbreviations: SS: Sjogren Syndrome, SARS-Cov: Severe Acute Respiratory Syndrome Coronavirus, COVID: Coronavirus Disease, NHL: Non Hodgkin Lymphoma, EULAR: European League Against Rheumatism, ACR: American College Of Rheumatology, PPI: Proton-Pump Inhibitor, EBV: Epstein-Barr Virus, HHV: Human Herpesvirus, ALL: Acute Lymphocytic Leukemia, AML: Acute Myeloid Leukemia
Introduction
Sjogren syndrome (SS) is a chronic and systemic autoimmune disorder, which is characterized by lymphocytic infiltration of exocrine glands (salivary and lacrimal), that causes drying of the eyes (keratoconjuctivitis sicca) and of the mouth (xerostomia) [1-3]. SS can cause extra-glandular organ manifestations, involving the skin, nervous system, kidneys, lungs and gastrointestinal tract that happens in 25% of the cases (3). The etiology of Sjogren syndrome is complicated, with environmental factors like viral infections being the main causative factor, where the salivary gland tissue is thought to be a latent site for the infection. Viruses like: Epstein-Barr virus (EBV) and human herpesvirus 6 (HHV6) [4], have similar domains to some antigenic epitopes on the La/SSB protein, causing antibody production [5]. Other etiological factors include genetic factors and smoking [4]. One of the complications with the heaviest burden, are hematologic complications like the transformation to Non Hodgkin Lymphoma, which happens in around 5% of patients with primary SS (pSS) and with a higher risk compared to the general population [6-7]. It has been speculated for many years about predicting factors to developing NHL in patients with pSS, with clinical predictors (persistent enlargement of salivary glands, lymphadenopathy, moderate/high disease activity, evaluated with ESSDAI), serological (low C4 serum levels, cryoglobulinemia, monoclonal gammopathy), histological (presence of germinal centers). These were the most consistent non-Hodgkin’s lymphoma/lymphoproliferative disease predictors [7-8]. Here by we present a case of a patient with pSS, that is complicated with a developing of NHL. We raise a question of whether infection with SARS-CoV-2 has a role in this malignant hematological transformation in this patient.
Case presentation
This is a 52 years old female patient, who presents in her GP clinic in 2017 complaining of tiredness, weakness and dryness of the eyes and mouth. In her medical history there is no data for autoimmune diseases, she suffers from hemorrhoids. Her family medical history shows nothing specific. In these conditions, the patient is not evaluated further and she gets treatment with artificial tears. In August 2021, the patient has similar complaints and she refers a swelling in the region of her parotid glands and arthralgia. She undergoes several laboratory examinations: CBC, biochemical panel and urine is normal. The immunological panel refers ANA (+) 1:640, C3 and C4, Rheumatoid factor (RF), SS-B anti ds DNA normal, SS-A (+). Then, a Schirmer test is performed which results positive 5 mm. Parotid gland ultrasound (Figure 1) show diffuse inhomogeneity with anechoic and hypoechoic areas. Then finally a “Lip Biopsy of Minor Salivary Gland Histopathology” was performed: Nodular lymphocyte infiltration (focal lymphocytic sialadenitis with a focus score ≥ 1 focus, defined as a number of lymphocytic foci per 4 mm2 of minor salivary gland tissue (one focus contains > 50 lymphocytes).
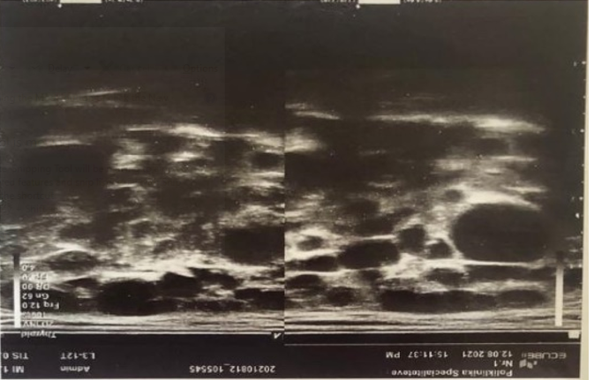
Figure 1: Diffuse inhomogeneity with anechoic and hypoechoic areas
In these conditions, according to the classification criteria for primary Sjogren’s Syndrome from the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR), the patient is diagnosed with Sjogren Syndrome and starts treatment with Plaquenil 200 mg/day, Medrol 4 mg, PPI, D3 vitamin and Calcium.
In October 2021, the patient has fever up to 38 grade Celsius, fatigue, myalgia. A RT-PCR was performed and SARS-CoV-2 genome was detected. It is noted a worsening of her Sjogren symptoms (dryness of the eyes and mouth, joint pain), but the patient didn’t need oxygen or hospitalization. She was followed up ambulatory and received treatment with Azithromycin 500 mg for three days, vitamins, antipyretics.
In April 2022, the patient complains of severe tiredness, dryness of the eyes and mouth, swollen glands like a ‘package’ in the neck region. She undergoes the following examinations:
Lymphatic system ultrasound: Multiple lymph nodes in the right parotid gland with a diameter of 30-17mm. Diffuse adenopathy is present in the right latero-cervical and supraclavicular region (dm 30-15mm), also in the left latero-cervical region involving the parotid gland and the posterior triangle of the neck. Tumoral markers: CEA, AFP, CA 19-9 (normal), CA 15-3 (↑) 86 (<30 units/mL) CA 125 (↑) 55 (0-35 units/mL), LDH (↑) 820 U/L (105-330 U/L). Infective panel: HIV (negative), Mantoux (negative), Toxoplasmosis IgM and IgG (negative), CMV IgG and IgM (normal), EBV IgM (normal) IgG (↑), HbsAg and AntiHCV (negative).
Thoracoabdominal CT: Diffuse adenopathy in conglomerate form: Supraclavicular, Subclavicular, Mediastinal, Abdominal, Inguinal, Femoral (from 20 to 40 mm) (Figure 2). Finally, lymph node biopsy is performed, where the morphological and immunohistochemical features are consistent with a diagnosis non Hodgkin lymphoma.
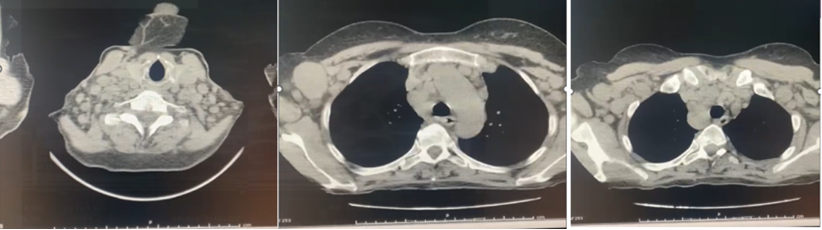
Figure 2: Diffuse adenopathy in conglomerate: Supraclavicular, Mediastinal, Abdominal, Inguinal, Femoral from 20 to 40 mm.
Discussion
We are presenting a clinical case with pSS, with a malignant hematological complication like NHL. In literature, it is known that NHL is one of the complications of pSS [8-9], that is estimated to have a risk 43.8 times higher than the healthy population [10]. Knowing this, the patients need to be monitored during the course of the disease, taking into consideration the predicting factors for developing NHL.
In our case, the patient has started showing aggravated symptoms of SS, in a time frame of 8 months after infection with SARS-CoV-2. Analyzing this evolution, a question was raised regarding this recently discovered virus, as a potential causative factor in the malignant hematological complication in this case. The patient presented in our clinic with parotid gland swelling for a year. Persistent enlargement of parotid glands is one of the predictive factors of developing NHL [11], and this should remain in our attention when we ask the question: ‘Has SARS-CoV-2 played a role in the hematological malignancy in predisposed patients (in this case, pSS)?’. There are some case reports in literature raising similar questions, presenting cases with hematological malignancies after infection with SARS-CoV-2. This raises the hypothesis for a possible connection between SARS-CoV-2 infection and hematological malignancies [12,13]. Cases that are discussed in literature are related to acute lymphocytic leukemia (ALL) and acute myeloid leukemia (AML), but there aren’t any case related to Non Hodgkin lymphoma.
Conclusion
Our case report suggests over a possible connection between NHL in a patient with pSS, several months after infection with Sars-Cov-2, and if this infection may have been a trigger for this complication. This is a hypothesis with no scientific evidence in literature, and it may be a random finding. Multiple other studies have to be executed in the future, to declare the effects of COVID-19 infection in patients with SS.
References
- Fox RI. (2005) Sjogren’s syndrome. Lancet. 366(9482): 321-331. [PubMed.]
- Mavragani CP, Moutsopoulos HM. (2014) Sjögren syndrome. CMAJ. 186(15): E579-E586. [Ref.]
- Vivino FB. (2017) Sjogren's syndrome: Clinical aspects. Clin Immunol. 182: 48-54. [PubMed.]
- Brito-Zerón P, Baldini C, Bootsma H, Bowman SJ, Jonsson R, et al. (2016) Sjögren syndrome. Nat Rev Dis Primers. 2: 16047. [PubMed.]
- Haaheim LR, Halse AK, Kvakestad R, Stern B, Normann O, et al. (1996) Serum antibodies from patients with primary Sjögren's syndrome and systemic lupus erythematosus recognize multiple epitopes on the La (SS-B) autoantigen resembling viral protein sequences. Scand J Immunol. 43(1): 115-121. [PubMed.]
- Stefanski AL, Tomiak C, Pleyer U, Dietrich T, Burmester GR, et al. (2017) The Diagnosis and Treatment of Sjögren's Syndrome. Dtsch Arztebl Int. 114(20): 354-361. [PubMed.]
- Nishishinya MB, Pereda CA, Muñoz-Fernández S, Pego-Reigosa JM, Rúa-Figueroa I, et al. (2015) Identification of lymphoma predictors in patients with primary Sjögren's syndrome: a systematic literature review and meta-analysis. Rheumatol Int. 35(1): 17-26. [PubMed.]
- Alunno A, Leone MC, Giacomelli R, Gerli R, Carubbi F. (2018) Lymphoma and Lymphomagenesis in Primary Sjögren's Syndrome. Front Med (Lausanne). 5: 102. [Ref.]
- Voulgarelis M, Dafni UG, Isenberg DA, Moutsopoulos HM. (1999) Malignant lymphoma in primary Sjögren's syndrome: a multicenter, retrospective, clinical study by the European Concerted Action on Sjögren's Syndrome. Arthritis Rheum. 42(8): 1765-1772. [PubMed.]
- Kassan SS, Thomas TL, Moutsopoulos HM, Hoover R, Kimberly RP, et al. (1978) Increased risk of lymphoma in sicca syndrome. Ann Intern Med. 89(6): 888-892. [PubMed.]
- De Vita S, Isola M, Baldini C, Goules AV, Chatzis LG, et al. (2022) Predicting lymphoma in Sjögren’s syndrome and the pathogenetic role of parotid microenvironment through precise parotid swelling recording. Rheumatology. keac470. [Ref.]
- Costa BA, da Luz KV, Campos SEV, Lopes GS, Leitão JPV, et al. (2022) Can SARS-CoV-2 induce hematologic malignancies in predisposed individuals? A case series and review of the literature. Hematol Transfus Cell Ther. 44(1): 26-31. [Ref.]
- Saini G, Aneja R. (2021) Cancer as a prospective sequela of long COVID-19. Bioessays. 43(6): e2000331. [Ref.]