>Corresponding Author : Nikolaos Andreas Chrysanthakopoulos
>Article Type : Research Article
>Volume : 2 | Issue : 1
>Received Date : 10 May, 2022
>Accepted Date : 19 May, 2022
>Published Date : 23 May, 2022
>DOI : https://doi.org/10.54289/JDOE2200103
>Citation : Chrysanthakopoulos NA, Vryzaki E. (2022) Investigation of Periodontal Disease Status in Acute Leukemia (Myeloid and Lymphoblastic) Greek Patients: A Case - Control Study. J Dent Oral Epidemiol 2(1): doi https://doi.org/JDOE2200103
>Copyright : © 2022 Chrysanthakopoulos NA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Research Article | Open Access
1Dental Surgeon, Oncologist (MSc), Specialized in Clinical Oncology, Cytology and Histopathology, Dept. of Pathological Anatomy, Medical School, University of Athens, Athens, Greece, Resident in Maxillofacial and Oral Surgery, 401 General Military, Hospital of Athens, Athens, Greece, PhD in Oncology (cand)
2MD, PhD, Department of Dermatology, Rio University Hospital of Patras, Greece, Running head: Periodontal Indices and Risk of Acute Hematopoietic Cancer,
*Corresponding author: Nikolaos Andreas Chrysanthakopoulos PhD, Dental Surgeon, Oncologist, Specialized in Clinical Oncology, Cytology and Histopathology, Dept. of Pathological Anatomy, Medical School, University of Athens, Athens, Greece, Resident in Maxillofacial and Oral Surgery, 401 General Military, Hospital of Athens, Athens, Greece
Abstract
Objectives: Oral manifestations in acute leukemia patients is a serious medical condition. The aim of the current research was to compare the periodontal condition in a group of acute, myeloid and lymphoblastic, leukemia (AM/ALL) patients with a control group of healthy individuals.
Methods: 98 patients with AM/ALL and 196 controls were selected. The clinical measurements used to diagnose periodontal condition concerned probing depths (PPDs), clinical attachment loss (CAL), gingival index (GI), bleeding on probing (BOP), and oral hygiene habits. The models of chi-square test and logistic regression were used to assess the possible differences between AM/ALL patients and controls.
Results: The mean ages were 65.7 ± 3.4 years and 66.2 ± 2.8 years for cases and controls, respectively. AM/ALL patients had worst periodontal parameters such as PPD (p = 0.052, OR =1.725, 95% CI = 0.995-2.00), tooth-brushing frequency (p = 0.046, OR = 0.581, 95% CI = 0.341-0.00), GI (p = 0.091, OR = 1.632, 95% CI = 0.924-2.88), and BOP (p = 0.011, OR = 2.05, 95% CI = 1.18-3.563), after adjustment for smoking, socio-economic and educational status, compared with healthy individuals.
Conclusion: Individuals with AM/ALL presented deeper periodontal pockets than healthy controls, worse gingival inflammation, and bleeding on probing than healthy controls and poor oral hygiene practices such as daily tooth brushing.
Keywords: Acute myeloid leukemia, acute lymphoblastic leukemia, adults, periodontal indices
Abbreviations: AM/ALL: Acute, Myeloid and Lymphoblastic, Leukemia, PPD: Periodontal Condition Concerned Probing Depths, CAL: Clinical Attachment Loss, GI: Gingival Index, BOP: Bleeding on Probing, PD: Periodontal Disease, RA: Rheumatoid Arthritis, AL: Acute Lymphoblastic, AML: Acute Myeloid Leukemia, CL: Chronic Lymphocytic, CML: Chronic Myeloid Leukemia, HL: Hodgkin Lymphomas, NHL: Non-Hodgkin Lymphomas, CI: Confidence Interval, WHO: World Health Organization, PI: Plaque Index
Introduction
Oral cavity health significantly reflects the whole organism health. Clinical signs and symptoms in oral cavity in many cases consist signs of hidden and serious systemic diseases and disorders [1]. Such diseases include immunologic diseases, hematologic conditions, systemic infections, endocrinopathies, and nutritional disorders. To be more specific diseases and disorders that have been identified include anemia, lichen planus, lupus erythematosus, pemphigus vulgaris, benign mucus membrane pemphigoid, Crohn disease, Bechet syndrome, diabetes mellitus, HIV-associated periodontal disease (PD), thrombocytopenia, leukemia, gastroesophageal reflux disease, bulimia and anorexia [2].
Moreover, an association between PD and other chronic diseases, such as type 2 diabetes mellitus [3], cardiovascular disease [4], respiratory diseases and COPD mainly [5], rheumatoid arthritis (RA) [6], inflammatory bowel disease [7], several types of cancer [8], etc. has been revealed in previous studies because similar mechanisms of tissue destruction have been described in these conditions.
Hematopoietic malignancies comprise 8-10% of all human malignancies, and include leukemias (Acute Lymphoblastic-AL, Acute Myeloid Leukemia-AML, Chronic Lymphocytic-CL, Chronic Myeloid Leukemia-CML), myelomas and lymphomas. Lymphomas are sub-classified as either Hodgkin lymphomas (HLs) or non-Hodgkin lymphomas (NHLs) [9].
AML is the most common hematopoietic malignancy among adults, affects about 4.2 per100,000 individuals, or more than 20,000 cases annually in the U.S [10]. The average age at the time of AML clinical diagnosis is about 65 years, and is more prevalent among males compared with females, with a ratio 5:3 [10].
It is characterized by clonal expansion of immature blast cells in the bone marrow and peripheral blood, that results in insufficient erythropoiesis and bone marrow damage [11,12]. AML’s etiology remains unknown, however several risk factors have been identified such as hematological diseases, myelodysplastic syndrome, myelofibrosis, polycythemia vera or thrombocythemia, and aplastic anemia. Other risk factors are congenital diseases such as Down and Bloom syndrome, whereas environmental exposures like radiation, chemical exposure (benzene), tobacco smoking, and previous exposure to cancer treatment (chemotherapy/ radiotherapy) agents have also been proposed [13-15].
The 5-year survival rate for younger patients is 50%, whereas the 5-year survival rate and the 3-year survival rate is 3-8% and 9-10% in 60 year old individuals and older, respectively [16,17]. ALL affects about 4,000 individuals in the U.S annually, the majority concerns individuals under the age of 18 as the main age of ALL diagnosis is between two and ten years of age, and its incidence overall in population is about 3.3 cases per 100,000 children. ALL represents 2 % of the lymphoid neoplasms and is more common in males than females [18,19]. ALL is a B- or T-lymphoblasts malignancy, histologically characterized by abnormal and unlimited proliferation of immature lymphocytes and their progenitors which ultimately leads to the substitution of bone marrow cells and other lymphoid organs resulting in the classic appearance of ALL [18].
Similar to AML, ALL’s etiology is unknown [20], however is more common in children with Bloom, Down and Li- Fraumeni syndromes, ataxia telangiectasia and Neurofibromatosis type I [21]. Environmental risk factors have also been implicated in its Etiology such as exposure to ionizing radiation, chemical agents(benzene), and previous exposure to cancer treatment (chemotherapy/radiotherapy) [21]. ALL has been earlier considered as a non-hereditary disease [21], however with large sequencing efforts growing numbers of predisposing genetic alterations have been recorded [22]. The possible causative role of pesticides or electromagnetic fields in ALL etiology remains unclear [23]. It has also been hypothesized that an abnormal immune response to a common infection may be implicated in ALL etiology [23].
The 5-year overall survival rate is greater than 85 % [24].
Oral lesions can be an important diagnostic indicator of acute leukemia [25], as frequently undiagnosed cases of leukemia refer to the dentist with signs and symptoms related to oral lesions [26], whose recognition can result in AM/ALL diagnosis [1]. In most AML patients oral manifestations are often the first presentation [26] and concern the subtypes of myeloid and monocytic/monoblastic leukemia [25]. Moreover, oral alterations can also occur in chronic leukemia [26], however, they differ from those revealed in acute leukemia [26] and are not considered specific. The most common oral manifestations of leukemia include petechiae or spontaneous bleeding in 56% of patients [25,26], mucosal ulceration in 53% [25,26], and diffuse or localized gingival enlargement in 36% [26,27], with or without necrosis [25]. The mentioned clinical signs are the most frequent leukemia initial diagnostic manifestations. Other oral manifestations include mucosal paleness, increased prevalence of dental caries [27], herpetic opportunistic infections and candidiasis [25], temporomandibular joint arthritis, osteolytic lesions in the mandible. Less commonly oral signs are palatal pigmentation, tooth pain and mobility, hemorrhagic bullae on the tongue, cracked lips, parotid swelling, and chin numbness [26].
Previous researchers have examined the periodontal conditions in patients with several chronic diseases and disorders. Some case reports have described severe periodontitis in patients with Crohn’s disease [28,29]. Flemming et al. [30] found a higher prevalence, but less severe periodontitis in individuals with inflammatory bowel disease. Miranda et al. [31] recorded that adolescents with juvenile idiopathic arthritis [JIA] present more periodontal attachment loss than healthy controls, in spite of similar plaque and marginal bleeding levels. Two similar studies showed that RA patients had more missing teeth and a higher percentage and significantly deeper pockets (6 mm or more) than the controls, but no differences in plaque and bleeding indices [32]. On the contrary, Walton et al. [33] reviewed the oral findings of JIA patients and concluded that they had a higher prevalence of dental caries and a higher bleeding index than controls. However, periodontitis outcomes, such as probing depth (PD) and clinical attachment loss (CAL), were not assessed. More recent articles have examined periodontal condition in several types of human malignancies [34-38], whereas studies that have examined the prevalence of periodontal status and indices in AM/ALL patients are not available [26]. Therefore, prospective and retrospective studies are required to further elucidate the association examined. The current research is the first in Greece that explored the possible differences, as no previous researches have been carried out.
The aim of the present retrospective case-control study was to assess the possible differences in periodontal condition between individuals who suffered from AML and ALL and healthy ones.
Materials and Methods
Study design
The current retrospective case – control research was carried out between May 2021 and December 2021. The study sample was evaluated according to EPITOOLS guidelines (https://epitools. ausvet.com.au) Determined with 95% Confidence Interval (CI) and desired power 0.8, whereas the age group was based on the World Health Organization (WHO) recommendations [39] for assessing periodontal condition incidence.
The patient group comprised 98 individuals who were attending two private medical and a dental practice. The diagnosis of AM / ALL made by the same physicians and derived from their medical files with a definitive diagnosis based on bone marrow examination aspiration and biopsy [40] and before the application of any treatment such as chemotherapy, radiation therapy, chemotherapy with stem cell transplant, and targeted therapy.
196 individuals were selected as controls, and their physicians ensured that they had no disease and were not taking any medication. Control group was selected by the friendly and collegial environment of cases, in an effort to control possible confounder effects such as smoking, SES, and educational status. Moreover, both groups were matched for gender and age, and were also selected from the same city population in an effort to select a representative study sample and to avoid or reduce possible selection biases. Therefore, control group was selected from individuals who were presented to routine health follow-up at the mentioned practices, between 2020 and 2021.
Cases and controls should not have had an history of any periodontal treatment, conservative or surgery in the last 6 months, or received systemic antibiotic regimens or a systematic treatment with glucocorticoids or immunosuppression agents within the previous 6 months. They should also have more than 15 teeth and periodontitis from stage I to IV [41]. Moreover, both groups should not be suffered from diseases such as cardiovascular disease, diabetes mellitus, rheumatoid arthritis, or any type of other malignancies. The mentioned diseases and disorders could have potential effects on oral tissues as confounders and could result in biased secondary associations.
The current study was not approved by authorized committees (Ministry of Health, etc.), as in Greece only experimental studies must be approved by the mentioned Authorities. All participants were informed about the aims and methods of the present study, and both gave their written consent to participate.
Research questionnaire and periodontal status examination
AML/ALL patients and healthy individuals filled in a modified Minnesota Dental School Medical Questionnaire [42], that included epidemiological variables such as age, gender, smoking status, SES, Educational status, and their past Medical / Dental history.
The same dentist re-examined clinically a randomly selected sample of 60 (20%) for assessing the intra-examiner variance after a time period of three weeks, and no differences were recorded between the 1st and the 2nd clinical assessment (Cohen's Kappa = 0.95). During this time period no oral hygiene instructions were given to the participants.
Periodontal condition was measured using PPD, CAL, bleeding on probing (BOP), and the gingival condition (Gingival Index, GI) at six sites in all teeth (disto-buccal, mesiobuccal, mid-buccal, mesio-lingual,disto-lingualand mid-lingual), apart from remaining roots and third molars, in all quadrants and the worst values of the indices recorded to the nearest1.0 mm and classified as dichotomous variables for each individual, using a type Williams probe with a controlled force of 0.2 N (DB764R, Aesculap AG & Co. KG, Tuttlingen, Germany), by the same dentist. PPD was dichotomously assessed as score 0: stage I [maximum PPD ≤ 4.0 mm] and score 1: stage II-IV [PPD ≤ 4.0 - ≥ 6.0 mm]. CAL severity was assessed as score 0: stage I [CAL: 1.0-2.0 mm], and score 1: stage II-IV [CAL: 3.00 - ≥ 5.0 mm] [41]. BOP absence was assessed as score 0 and presence as score 1, respectively [43], and the severity of gingivitis (GI) classified as score 0: normal situation of gingival tissue/mild inflammation, insignificant change in colour and oedema, absence of bleeding on probing, which corresponds to Löe [44] classification as score 0 and 1, and -score 1: moderate inflammatory reaction with presence of redness, oedema, glazing and bleeding on probing/severe inflammatory reaction with presence of significant redness, oedema, ulceration and tendency to spontaneous bleeding, which corresponds to Löe classification as score 2 and 3.
Data Statistical analysis
Females, non-smokers, low socioeconomic [income/monthly Equivalent to or less than 1,000 €] and educational level [graduated from Elementary/High School], irregular daily tooth-brushing Daily [≤1/daily], and controls were coded as 0. Age groups distribution was coded as 0, 1, 2, and 3 for ages 47-50, 51-60, 61-70 and 71+, respectively. The univariate analysis (chi-square) model was carried out to assess the associations between the independent variables examined and cases/ controls, separately. Multivariate logistic regression analysis model was carried out to estimate the associations between the dependent variable, AML/ALL, and independent ones using the Enter and Stepwise methods. Unadjusted and Adjusted Odds Ratios [OR's] and 95% [Confidence Interval] CI were also estimated. The statistical model Cohran’s and Mantel-Haenszel’s was applied to control possible confounders, such as smoking, SES and educational status. Statistical analysis was performed by SPSS statistical package [SPSS PC20.0, SPSS, Inc., Chica-go, IL, USA], and a p value less than 5% [p< 0.05] was considered to be statistically significant.
Results
The mean ages were 65.7 ± 3.4 years and 66.2 ± 2.8 years for cases and controls, respectively. Univariate model was used to compare indices between cases and controls for categorical variables. The outcomes showed that moderate/severe gingivitis (GI) (p = 0.006) and BOP (p = 0.001) in cases were statistically significant greater compared to healthy individuals (Table 1). The same table presents unadjusted OR’s and 95% CI. After performing logistic regression model (Enter Method - Step 1a) inadequate tooth brushing (p = 0.046), deep periodontal pockets (p = 0.025), moderate/severe gingivitis (p = 0.049), and BOP (p = 0.013) were statistically significant different between cases and controls (Table 2). The final step (Wald Method) confirmed the out-comes of the previous method, as the same indices were different between cases and healthy individuals in statistically significant level. Adjusted OR’s and 95% CI are also shown in the same Table. (Tables 3) Presents the outcomes after performing the statistical model Cohran’s and Mantel-Haenszel’s for controlling possible confounders, such as smoking, SES and educational status.
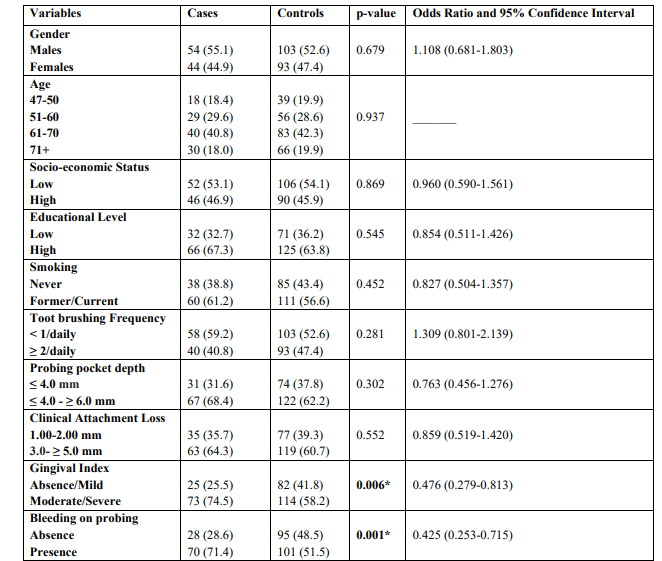
Table 1: Univariate analysis of cases and controls regarding each independent variable examined
* p-value statistically significant
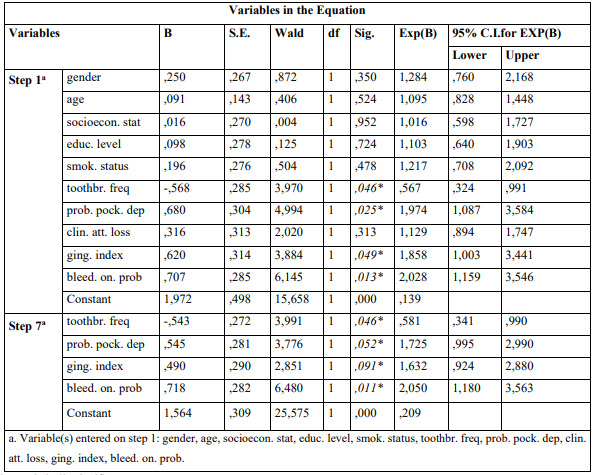
Table 2: Presentation of association between parameters examined and AM/ALL according to Enter (first step-1a) and Wald (final step-7a) methods of Multivariate Logistic Regression Analysis Model
* p-value statistically significant
Discussion
The aim of the current research was to investigate whether the periodontal status in AML/ALL patients was worse or not, compared with periodontal status in healthy individuals. The most frequent indices used for estimating periodontal status are clinical indices such as number of tooth loss/remaining teeth, PPD, CAL, Gingival Index (GI), Plaque Index (PI), BOP, Bleeding Point Index, Alveolar Bone Loss (ABL), etc. [45]. The outcomes of the current study showed that PPD in cases group was statistically significant greater than in control group, finding that remained after adjusting for possible confounders as smoking, SES and educational status. PPD reflects the destructive process of a chronic inflammatory response and is an indicator for estimating the severity of PD [46]. Leukemic patients according to radiographic examinations, have shown loss of lamina dura, changes in periodontal space, resorption of the alveolar bone, and destruction of the bone structure [47]. All these symptoms are related to leukemic cell infiltrations [47,48]. Similarly, gingival inflammation as presented by GI showed worse levels in AML/ALL patients compared to healthy individuals. This finding is in accordance with the pathological physiology of the disease, as gingival hyperplasia especially in interdental papilla or gingival margins is common clinical sign in acute cases [1]. Gingival enlargement and ulcerations are a result of inflammation, neutropenia or direct infiltration of immature (blasts) proliferating leukocytes or be secondary to thrombocytopenia and immunodeficiency and it could be localized or generalized [49-52]. This infiltration leads to an increase in gingival thickness and formation of pseudo-pockets, resulting in secondary inflammatory infiltration [50]. The continuous trafficking of myeloid cells in specialized post-capillary venules accounts for egress of these cells from the circulation into the tissues at the sites of gingivitis or periodontitis [53]. Leukemic gingival infiltration is not observed in edentulous individuals, suggesting that local irritation and trauma of the teeth are associated in the pathogenesis [54]. The gingival findings are reported to be partially dependent on the inflammation of the tissues [55]. On the other hand, chronic leukemic cases have lesser infiltration, and the gingiva would be paler [49].
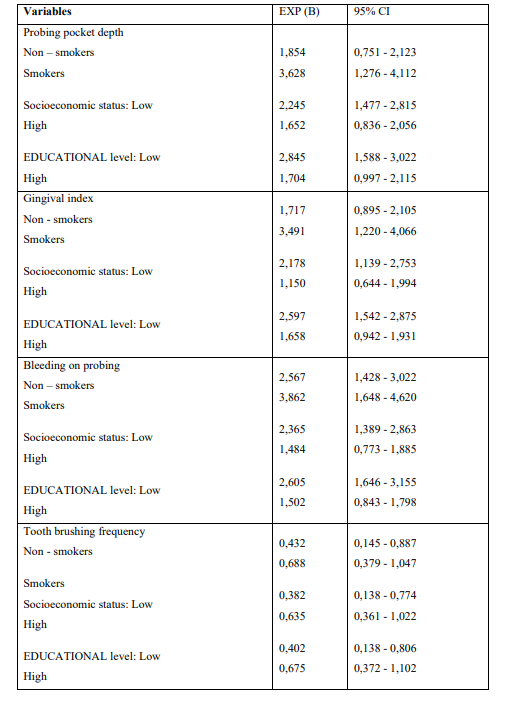
Table 3: Application of Cohran’s and Mantel-Haenszel’s statistical method for controlling Confounders
Gingival enlargement is more frequent in AML patients than in other types of leukemia, and it occurs more frequently in the M4 and M5 subtypes [56,57].
Gingival bleeding, as expressed by BOP is another periodontal status index that was found to be worsen in AML/ALL patients than healthy individuals. This observation can be attributed to the fact that gingival bleeding is caused by severe thrombocytopenia when platelet number is lower than 20,000 cells/mm3, that is a clinical sign of acute leukemia [58].
BOP index reflects the host’s vascular response in terms of hyperemia, the capillaries’ dilation and increased blood flow in the inflammation region. BOP is a widely used criterion to diagnose gingival inflammation, however it has been suggested that periodontal pockets with a probing depth of greater than or equal to 5.00 mm showed a significantly higher incidence of BOP [59]. The outcomes of the current study also showed that oral hygiene habits were significantly different between cases and controls and that AML/ALL-individuals had worse oral hygiene.
AML is the most common hematopoietic malignancy among adults, the average age at the time of AML clinical diagnosis is about 65 years and is more prevalent among males compared with females. Moreover, male gender, and advanced age increase the risk for ALL development [60]. Despite the fact that older individuals are at higher risk for AML development, age is considered as a confounder. Similarly, older individuals are at higher risk for initiation and progression of PD indices [61]. The difference regarding the parameter of age and gender between cases and controls was not statistically significant in the current report.
Smoking is a known carcinogenesis risk factor and can affect leukemia risk [61,62]. In addition, smoking is a major risk factor for PD development and progression [61,63]. No difference was recorded regarding smoking habits between cases and controls, whereas smoking is another crucial confounder.
SES/educational status are known crucial confounders. Moreover, it has also been proposed that high-educated and SES individuals take care of their own oral hygiene more than low-educated and SES ones and could prevent diseases that are associated with PD [64]. No significant differences were recorded between AM/ALL individuals and healthy ones regarding the educational level/SES in the present research.
In the international literature epidemiological studies that have examined the possible differences of the mentioned indices between AM/ALL patients and healthy individuals have not been carried out, since most of those available refer only to the description of isolated clinical cases. Only Busjan et al. [65], in a cross-sectional study, compared the oral mucosa lesions of 39 leukemia patients with those of 38 healthy patients, observing that 68% of the leukemia patients presented oral mucosa lesions, gingival hyperplasia being this study the most important finding. Significantly higher GI in children with ALL, compared to children from the control group, was observed despite the better oral hygiene [66].
A recent study highlighted that, whereas there was no significant association between gingival bleeding index and suffering from AML, there could be a certain trend between increased bleeding and AML. A higher caries prevalence in leukemia patients was shown in the same study whereas the periodontal parameters were poorer in leukemia patients [67].
Kapoor et al. revealed a lower prevalence of dental caries, good oral hygiene and mild gingivitis in children with ALL when compared to healthy children [68]. A systematic review with meta-analysis, in which dental caries and periodontal diseases were assessed using the following standardized parameters, respectively: mean number of decayed, missing and filled teeth (DMFT), and presence of marginal inflammation (gingivitis) or clinical attachment loss (periodontitis) resulted in no significant outcomes as data was too few to run a meta-analysis [69].
SES, educational status and smoking were the main reason why the Cochran and Mantel-Haenszel model was carried out in an effort to clarify if possible significant differences between AM/ALL patients and healthy individuals regarding the PD indices examined could be attributed to those epidemiological parameters. The model showed that smoking, educational and SES were not con-founders of periodontal status indices examined. The current retrospective case-control study has several limitations that must be taken into account. Case-controls studies are susceptible to selection, recall, random, referral bias and their outcomes must be adjusted for known and unknown con-founders which can lead to biased secondary associations regarding the indices examined. In contrary, prospective cohort studies design can control confounding biases.
Strengths of the current study were the adequate and representative study sample and that it was a matched case-control study, as was used randomly selected population-based controls as were selected from healthy individuals derived from cases environment, methodology that warrants interval validity. It is obvious that more studies, especially prospective are needed to confirm those findings.
Conclusion
Patients suffering from AM/ALL presented deeper periodontal pockets than healthy controls, worse gingival inflammation and bleeding on probing than healthy controls and poor oral hygiene practices such as daily tooth brushing.
Conflict of interest and source of funding statement: The authors declare that they have no conflict of interest
References
- Im HJ. (2018) Current treatment for pediatric acute myeloid leukemia. Blood Res. 53: 1-2. [Ref.]
- Chi AC, Neville BW, Krayer JW, Gonsalves WC. (2010) Oral Manifestations of Systemic Disease. Am Fam Physician. 82(11): 1381-1388. [Ref.]
- Kim J, Amar S. (2006) Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 94: 10-21. [PubMed.]
- Beck JD, Offenbacher S. (2005) Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 76: 2089-2100. [PubMed.]
- Scannapieco FA, Bush RB, Paju S. (2003) Associations between Periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic review. Ann Periodontol. 8: 54-69. [Ref.]
- Ortiz P, Bissada N, Palomo L, Han YW, Al-Zahrani MS, et al. (2009) Periodontal therapy reduces the severity of active rheumatoid arthritis in patients treated with or without tumornecrosis factor inhibitors. J Periodontol. 80: 535-540. [PubMed.]
- Yang-yang She, Xiang-bo Kong, Ya-ping Ge, Zhi-yong Liu, Jie-yu Chen, et al. (2020) Periodontitis and inflammatory bowel disease: a meta-analysis. BMC Oral Health. 20: 67 [PubMed.]
- Fitzpatrick SG, Katz J. (2010) The association between Periodontal disease and cancer: a review of the literature. J Dent. 38: 83-95. [Ref.]
- Wu Y, Shi X, Li Y, Xia Ju, Gu Y, et al. (2020) Hematopoietic and lymphatic cancers in patients with periodontitis: a systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal. 25: e21-e28. [Ref.]
- Vakiti A, Mewawalla P. (2022) Acute Myeloid Leukemia. StatPearls Publishing. [Ref.]
- Bain BJ, Béné MC. (2019) Morphological and Immunophenotypic Clues to the WHO Categories of Acute Myeloid Leukaemia. Acta Haematol. 141: 232-244. [Ref.]
- Naymagon L, Marcellino B, Mascarenhas J. (2019) Eosinophilia in acute myeloid leukemia: Overlooked and Underexamined. Blood Rev. 36: 23-31. [Ref.]
- Khwaja A, Bjorkholm M, Gale RE, Levine RL, Jordan CT, et al. (2016) Acute myeloid leu-kemia. Nat Rev Dis Prim. 2: 16010. [Ref.]
- Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. (2019) Epidemiology of acute mye-loid leukemia: Recent Progress and enduring challenges. Blood Rev. 36: 70-87. [PubMed.]
- Bizzozero OJ, Johnson KG, Ciocco A. (1996) Radiation-related leukemia in Hiroshima and Nagasaki, 1946-1964. I. Distribution, incidence and appearance time. New Eng J Med. 274: 1095-1101. [PubMed.]
- Lerch E, Espeli V, Zucca E, Leoncini L, Scali G, et al. (2009) Prognosis of Acute myeloid leukemia in the general population: data from southern Switzerland. Tumori. 95: 303-310. [PubMed.]
- Alibhai SM, Leach M, Minden MD, Brandwein J. (2009) Outcomes and quality of care in acute myeloid leukemia over 40 years. Cancer. 115: 2903-2911. [PubMed.]
- Roberts KG. (2018) Genetics and prognosis of ALL in children vs adults. Hematology Am Soc Hematol Educ Program. 30: 137-145. [PubMed.]
- Dinner S, Liedtke M. (2018) Antibody-based therapies in patients with acute lymphoblastic leukemia. Hematol Am So. Hematol Educ Program. 30: 9-15. [PubMed.]
- Hunger SP, Mullighan CG. (2015) Acute Lymphoblastic Leukemia in Children. New Eng J Med. 373: 1541-1552. [PubMed.]
- Vrooman LM, Silverman LB. (2016) Treatment of Childhood Acute Lymphoblastic Leukemia: Prognostic Factors and Clinical Advances. Curr Hematol Malig Rep. 11: 385-394. [PubMed.]
- Pui CH, Nichols KE, Yang JJ. (2019) Somatic and germline genomics in paediatric acute lymphoblastic leukaemia. Nat Rev Clin Oncol. 16(4): 227-240. [PubMed.]
- Inaba H, Greaves M, Mullighan CG. (2013) Acute lymphoblastic leukaemia. Lancet. 381: 943-1955. [Ref.]
- Ceppi F, Cazzaniga G, Colombini A, Biondi A, Conter V. (2015) Risk factors for relapse in childhood acute lymphoblastic leukemia: prediction and prevention. Expert Rev Hematol. 8: 57-70. [Ref.]
- Moazzez AH, Alvi A. (1998) Head and neck manifestations of AIDS in adults. Am Fam Physician. 57(8): 1813-1822. [Ref.]
- U.S. Department of Health and Human Services. (2000) Oral health in America: a report of the Surgeon General. Rockville, Md.: U.S. Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health. [PubMed.]
- Gonsalves WC, Chi AC, Neville BW. (2007) Common oral lesions: Part I. Superficial mucosal lesions. Am Fam Physician. 75(4): 501-507. [PubMed.]
- Lamster I, Sonis S, Hannigan A and Kolodkin A. (1978) An association between Crohn’s disease, Periodontal disease and enhanced neutrophil function. J Periodontol. 49: 475-479. [Ref.]
- Engel LD, Pasquinelli KL, Leone SA, Moncla BJ, Nielson KD, et al. (1988) Abnormal lymphocyte profiles and leukotriene B4 status in a patient with Crohn’s disease and severe periodontitis. J Periodontol. 59: 841-847 [Ref.]
- Flemmig TF, Shanahan F, Miyasaki KT. (1991) Prevalence and severity of Periodontal disease in patients with inflammatory bowel disease. J Clin Periodontol. 18: 690-697. [PubMed.]
- Miranda LA, Fischer RG, Sztajnbok FR, Figueredo CMS, Gustafsson A. (2003) Periodontal conditions in patients with juvenile idiopathic arthritis. J Clin Periodontol. 30(11): 969-974. [PubMed.]
- Mercado FB, Marshall RI, Klestov AC, Bartold PM. (2001) Relationship between rheumatoid arthritis and periodontitis. J Periodontol. 72(6): 779-787. [PubMed.]
- Walton AG, Welbury RR, Thomason JM, Foster HE. (2000) Oral health and juvenile idiopathic arthritis: a review. Rheumatol. 39: 550-555. [Ref.]
- Chrysanthakopoulos NA, Oikonomou A. (2017) A Case-Control Study of Periodontal Condition in Gastric Cancer Patients. Stomatol Dis Sci J. 1-7. [PubMed.]
- Chrysanthakopoulos NA. (2018) Periodontal Disease Indices in Pre-treatment Patients with the Most Frequent Types of Cancer. Mathews J Dent. 3(1): 1-9. [PubMed.]
- Chrysanthakopoulos NA. (2018) A Case-Control Study to Investigate an Association Between Lung Cancer Patients and Periodontal Disease. Sci Arch Dent Sci. 1(1): 36-42. [Ref.]
- Chrysanthakopoulos NA, Chrysanthakopoulos PA. (2019) Periodontal Condition in Patients with Glioblastoma: A Case- Control Study. J Oral Dent Health Res. 1(1): 1-6. [Ref.]
- Chrysanthakopoulos NA, Oikonomou AA. (2020) Examination of Periodontal Status in Chronic Obstructive Pulmonary Disease Greek Adults: A Case-Control Study. ARC J Dent Sci. 5(4): 1-12. [Ref.]
- World Health Organization. (1997) Oral health surveys: basic methods. Geneva: World Health Organization. [Ref.]
- Béné MC, Grimwade D, Haferlach C, Haferlach T, Zini G. (2015) Leukemia diagnosis: today and tomorrow. Europ J Haematol. 95: 365-373. [Ref.]
- Tonetti MS, Greenwell H, Kornman KS. (2018) Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Clin Periodontol. 45: S149-S161. [Ref.]
- Molloy J, Wolff LF, Lopez-Guzman A, Hodges JS. (2004) The Association of Periodontal disease parameters with systemic medical conditions and tobacco use. J Clin Periodontol. 31: 625-632. [PubMed.]
- Peikert SA, Mittelhamm F, Frisch E, Vach K, Ratka Krüger P, et al. (2020) Use of Digital Periodontal data to compare periodontal treatment outcomes in a practice-based research network (PBRN): a proof of concept. BMC Oral Health. 220: 297. [PubMed.]
- Löe H. (1967) The Gingival Index, the Plaque Index, and the Retention Index Systems. J Periodontol.38: 610-616. [PubMed.]
- Page RC, Eke PI. (2007) Case definitions for use in Population-based surveillance of periodontitis. J Periodontol. 78: 1387-1399. [PubMed.]
- Zimmermann H, Hagenfeld D, Diercke K, El-Sayed N, Fricke J, et al. (2015) Pocket depth and Bleeding on Probing and their associations with dental, lifestyle, socioeconomic and blood variables: a cross-sectional, multicenter feasibility study of the German National Cohort. BMC Oral Health. 15: 7. [PubMed.]
- Brazelton J, Louis P, Sullivan J, Peker D. (2014) Temporomandibular joint arthritis as an initial presentation of acute myeloid leukemia with myelodysplasia-related changes: a report of an unusual case. J Maxillofac Surg. 72: 1677-1683. [PubMed.]
- AlHazmi BA. (2021) Leukemia and periodontal health. J Pak Dent Assoc. 30(1): 61-65. [PubMed.]
- Little JW, Falace DA, Miller CS, Rhodus NL. (1997) Disorders of White blood cells. In: Dental Management of the Medically Compromised Patient.St.Louis, Missouri: Elsevier, Mosby. 373-395 [PubMed.]
- Fernandes KS, Gallottini M, Castro T, Amato MF, Lago JS, et al. (2018) Gingival leukemic infiltration as the first manifestation of acute myeloid leukemia. Spec Care Dent.38: 160-162. [PubMed.]
- Adisen MZ, Yilmaz S, Misirlioglu M. (2015) Diagnosis of acute myeloid leukemia in a dental hospital; report of ˇa case with severe gingival hypertrophy. Niger J Clin Pr. 18: 573. [Ref.]
- George N, Santhosh VC, Kumar H, Gopal S. (2015) Gingival enlargement in myelodysplastic syndrome. J Indian Soc Periodontol. 19: 687-689. [Ref.]
- Shankarapillai R, Nair MA, George R, Walsh LJ. (2010) Periodontal and gingival parameters in young adults with acute myeloid leukaemia in Kerala, South India. Oral Health Prev Dent. 8: 395-400. [Ref.]
- Reenesh M, Munishwar S, Rath SK. (2012) Generalised Leukaemic Gingival Enlargement: A Case Report. J Oral Maxillofac Res. 3: 5. [PubMed.]
- Choudhry K, Tandon S, Lamba AK, Faraz F. (2018) Leukemic gingival enlargement: A Case Report and review of literature. J Oral Maxillofac Surg Med Pathol. 22: S77- S81. [PubMed.]
- Wu J, Fantasia JE, Kaplan R. (2002) Oral manifestations of Acute Myelomonocytic leukemia: a case report and review of the classification of leukemias. J Periodontol. 73: 664-668. [Ref.]
- Felix DE, Lukens J. (1986) Oral symptoms as a chief sign of Acute Monoblastic leukemia: report of case. J Am Dent Assoc. 113: 899-900. [PubMed.]
- Epstein JB, Vickars L, Spinelli J, Reece D. (1992) Efficacy of Chlorhexidine and nystatin rinses in Prevention of Oral complications in leukemia and bone marrow transplantation. Oral Surg Oral Medic Oral Path. 73: 682-689. [PubMed.]
- Lang NP, Joss A, Orsanic T, Gusberti FA, Siegrist BE. (1986) Bleeding on probing. A Predictor for the progression of periodontal disease? J Clin Periodontol. 13: 590-596. [PubMed.]
- Greenlee RT, Hill-Harmon MB, Murray T, Thunm M. (2001) Cancer statistics, 2001. CA: A Canc J Clinic 51: 15-36. [Ref.]
- Newmann MG, Takei HH, Carranza F Carranza FA. (2006) Epidemiology of gingival and periodontal diseases. In, editor. Textbook of Carranza’s Clinical Period Ontology. St Louis. Missouri: Saunders. 86-91. [PubMed.]
- Belson M, Kingsley B, Holmes A. (2007) Risk factors for acute leukemia in children: a review. Environ Health Rerspect. 115: 138-145. [PubMed.]
- US Department of Health and Human Services. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. The Health Consequences of Smoking. [Ref.]
- Astrøm AN, Rise J. (2001) Socio-economic differences in patterns of health and oral health behaviour in 25-year-old Norwegians. Clin Oral Investig. 5: 122-128. [Ref.]
- Busjan R, Hasenkamp J, Schmalz G, Haak R, Trümper L, et al. (2018) Oral health status in adult patients with Newly diagnosed acute leukemia. Clin Oral Investig. 22(1): 411-418. [Ref.]
- Pels E, Mielnik-Błaszczak M. (2012) Oral hygiene in Children suffering from acute lymphoblastic leukemia living in rural and urban regions. Ann Agricult Environ Med. 19(3): 529-533. [Ref.]
- López-Valverde N, López-Valverde A, Gómez-de Diego R, Ramírez JM, Flores-Fraile J, et al. (2019) Gingival hyperplasia as an Early Manifestation of acute myeloid leukemia. A retrospective review. J Clin Exp Dent. 11(12): e1139-1142. [Ref.]
- Kapoor G, Goswami M, Sharma S, Mehta A, Dhillon JK. (2019) Assessment of oral health status of children with Leukemia: A cross-sectional study. Spec Care Dentist. 39(6): 564-571. [Ref.]
- Daniela P, Angst M, Maier J, Santos Nogueira RD, Manso IS, et al. (2020) Oral health status of patients with leukemia: a systematic review with meta-analysis. Arch Oral Biol.120: 104948. [PubMed.]