>Corresponding Author : Darrell O Ricke
>Article Type : Research Article
>Volume : 2 | Issue : 2
>Received Date : 15 May, 2022
>Accepted Date : 27 May, 2022
>Published Date : 31 May, 2022
>DOI : https://doi.org/10.54289/JVVD2200108
>Citation : : Ricke DO. (2022) Vaccines Associated Cardiac Adverse Events, Including SARS-Cov-2 Myocarditis, Elevated Histamine Etiology Hypothesis. J Virol Viral Dis 2(2): doi https://doi.org/10.54289/JVVD2200108
>Copyright : © 2022 Ricke DO. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Research Article | Open Access
Molecular BioInsights, Winchester, MA 01890
*Corresponding author: Darrell O. Ricke, Molecular BioInsights, Winchester, MA 01890
Abstract
Background: Rare cardiac adverse events are reported post vaccinations. For the SARS-CoV-2 mRNA Spike vaccines, higher numbers of these cardiac adverse events are being reported with myocarditis disproportionately occurring in younger males. The etiology of these cardiac adverse events associated with vaccines including SARS-CoV-2 is unknown. The etiology of the higher frequency of these cardiac adverse events temporally associated with SARS-CoV-2 mRNA Spike vaccines is also unknown.
Aim: Data mine vaccine associated cardiac adverse events to gain insights into COVID-19 mRNA associated myocarditis and pericarditis adverse events.
Methods: All adverse events, with a focus on cardiac adverse events, were summarized from the Vaccine Adverse Event Reporting System (VAERS) for all vaccines from 1990 to April 1, 2022.
Results: Analogous patterns of cardiac adverse events were observed for multiple unrelated vaccines with occurrences proportional to vaccine reactogenicity level defined all adverse events. This article proposes the hypothesis that innate immune responses to vaccines cause elevated histamine levels post vaccination; the histamine level reached may exceed the vaccinees’ histamine tolerance level for several days, with the histamine level likely correlating with the vaccine reactogenicity level. Further, it is proposed that the elevated histamine level is causative for the reported cardiac adverse events. For myocarditis and pericarditis reported adverse events, the elevated histamine levels may induce cardiac capillary pericyte vasoconstrictions followed by localized ischemia and anoxia; this is followed by the release of troponin from myocyte cells affected by anoxia. This hypothesis is supported by the temporal onset timing of adverse events reported following SARS-CoV-2 mRNA Spike vaccinations in VAERS.
Conclusion: Onset of cardiac adverse events immediately following vaccinations for multiple unrelated vaccines may implicate elevated histamine levels from immune responses as causative for these adverse events.
Relevance for patients. An etiology model for cardiac adverse events temporally associated with vaccination is proposed. If validated, this model identifies possible candidate treatments for evaluation with the potential to reduce the severity and frequencies of these cardiac adverse events for vaccinees.
Keywords: COVID-19, adverse events, histamine intolerance, myocarditis, pericarditis, tachycardia
Abbreviations: VAERS: Vaccine Adverse Event Reporting System, AE: Adverse Events, HIT: Histamine Intolerance, DAO: Diamine Oxidase
Graphical abstract
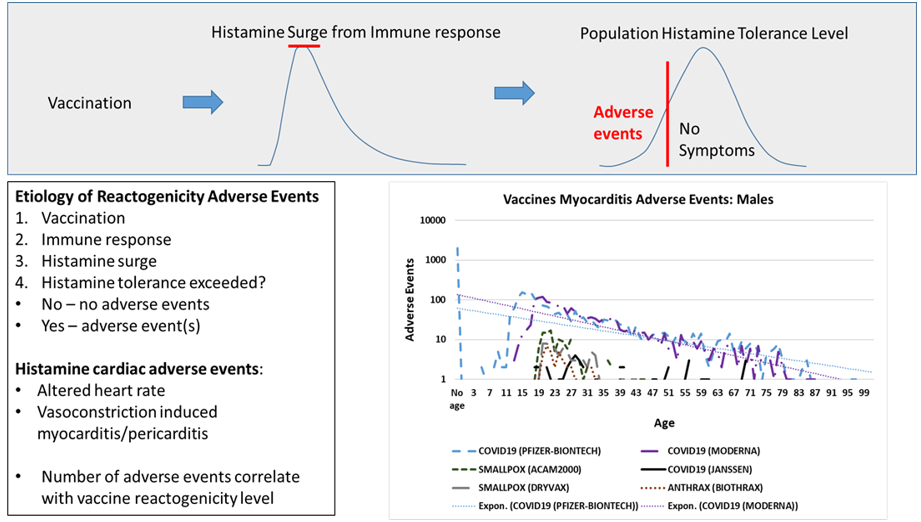
Introduction
Vaccinations protect vaccinees against multiple viral and bacterial infectious diseases. Some vaccinees experience mild adverse events (AE), multiple AE, or serious AE. Immediate short-term reactions are referred to as vaccine reactogenicity. The amount of reactogenicity varies by each specific vaccine. Very rare instances of myocarditis have been reported associated with vaccinations including tetanus [1], triple immunizations [2], etc. High numbers of COVID-19 cardiac adverse events, including myocarditis [3–7], pericarditis [8–13], and tachycardia [14–20] are being reported by COVID-19 vaccinees. Myocarditis has been significantly associated with both SARS-CoV-2 mRNA Spike vaccines (mRNA-1273 Moderna and BNT162b2 Pfizer/BioNTech) [21]. A retrospective case series including 21 COVID-19 vaccine associated myocarditis patients found elevated troponin levels in 100% of the 14 hospitalized patients [22]. Elevated troponin levels are a signature of some level of cardiac myocyte cell death. A Danish study of 4,931,775 individuals found absolute rates for myocarditis or myopericarditis at 1.4 per 100,000 for the BNT162b2 vaccine and 4.2 per 100,000 for mRNA-1273 vaccine [23]. A Nordic residents study of 23,122,522 individuals detected 5.55 (95% CI, 3.70-7.39) events per 100,000 vaccinees after the second dose of BNT162b2 and 18.39 (9.05-27.72) events per 100,000 vaccinees after the second dose of mRNA-1273 with similar estimates for pericarditis [24]. A survey of hospitalized Israeli Defense Forces military personnel reported an incidence rate of 5.07 per 100,000 [25]. A study of 404,407 Israeli adolescents reported 8.09 myocarditis cases per 100,00 for males and 0.69 cases per 100,000 for females [26]. A Hong Kong study of 224,560 adolescents reported incidence rates of 3.12 (1.25-6.42) and 22.15 (15.51-30.67) per 100,000 for the first and second dose of BNT162b2 vaccine [27]. The etiology of vaccine associated cardiac events is unknown.
In COVID-19 patients with myocarditis, vasoconstrictions associated with clamped pericyte cells has been proposed as the initial step in myocarditis [28]. Pericyte cell clamping was proposed to be caused possibly by either direct SARS-CoV-2 infection or by elevated histamine levels [28].
Cardiac responses to the β-imanazolylethylamine derivative of histamine was described by Dale & Laidlaw [29]. These cardiac responses include altered blood-pressure, constriction of coronary arterioles, constriction of pulmonary arterioles, vasodilation in limbs, altered heart rate, and heart failure varying by dose and animal species [29]. In pythons, histamine induces postprandial tachycardia through a direct effect on cardiac histamine H2-receptors [30]. See Wolff & Levi [31] for review histamine and cardiac arrhythmias. These cardiac adverse symptoms are also observed in some individuals with histamine intolerance (HIT) [32].
The Hypothesis
Innate immune responses to vaccination are implicated by consistent immediate onset patterns of cardiac adverse events shared by unrelated vaccines reported in Vaccine Adverse Event Reporting System (VAERS). Elevated histamine levels from innate immune responses are hypothesized herein as causative for these reported cardiac adverse events. For affected vaccinees, these cardiac adverse events are proposed to occur when the amount of histamine released from innate immune response exceeds their histamine tolerance level causing temporary histamine intolerance. The reported cardiac adverse events can be grouped into two subclasses. In the first subclass, elevated histamine level is associated with altered heart rate, including chest pain, palpitations, tachycardia, etc. Myocarditis and pericarditis represent a second subclass hypothesized to be initiated by histamine induced contraction of cardiac capillary pericyte cells; extended contraction of pericyte cells can result in vasoconstrictions followed by localized myocyte anoxia (cell death due to lack of oxygen). Localized myocyte anoxia is consistent with observed increases in troponin levels associated with myocarditis and pericarditis.
This model of elevated histamine induced vaccine cardiac adverse events generalizes to all vaccines with reported cardiac associated adverse events. The model of cardiac adverse events induced by elevated histamine level directly suggests multiple candidate prophylactic combined with therapeutic treatment options for evaluation that have the potential to reduce the incidence rate and severity of cardiac adverse events associated with vaccines. Therapeutically, these treatments may reduce the cardiac tissue damage caused by proposed vasoconstrictions and localized myocyte anoxia.
Materials and Methods
The Vaccine Adverse Event Reporting System (VAERS) database [33] was utilized for cardiac adverse events from 1990 to April 1, 2022. Reports of cardiac adverse events were identified by vaccine name or type, age, gender, onset day post vaccination, and vaccine dose. The following cardiac related adverse events were extracted: Acute myocardial infarction, Arrhythmia, Atrial fibrillation, Atrial flutter, Bradycardia, Cardiac arrest, Cardiac disorder, Cardiac failure, Cardiac flutter, Cardio-respiratory arrest, Chest discomfort, Chest pain, Electrocardiogram abnormal, Electrocardiogram ST segment elevation, Heart rate abnormal, Heart rate decreased, Heart rate increased, Heart rate irregular, Ischaemic stroke, Musculoskeletal chest pain, Myocarditis, Myocardial infarction, Myocardial necrosis marker, Palpitations, Pericarditis, Pericardial effusion, Pulmonary embolism, Sinus tachycardia, Tachycardia, Troponin increased, Troponin I increased. The downloaded data include all adverse events reported from 1990 to April 1, 2022. The Ruby program, named vaers_slice.rb [34], was used to tally selected reported vaccine adverse events by vaccine, age, and day of onset. The vaers_slice.rb program takes as input a list of one or more adverse events to characterize; these adverse events are summarized from the yearly VAERS Symptoms, Vax, and Data files from 1990 to 2022. The output from vaers_slice.rb consists of five reports: summaries by vaccine, summaries by age of onset of symptoms, summaries by day of onset of symptoms, and two summaries of additional symptoms reported (selected symptoms and all other symptoms). A similar program, named vaers_tally.rb, was developed to summarize all adverse events across all vaccines. Microsoft Excel was used create figures.
Results
All of the VAERS adverse events from 1990 to April 1, 2022, are summarized in the supplemental data tables named V_matrix (by vaccine name) and Vaccine_matrix (by vaccine type). Individual and combined symptoms report for selected cardiac related adverse events summarized by vaers_slice.rb are included in the supplemental data as individual Excel worksheets. Cardiac associated adverse events reported in VAERS are summarized in Table 1 for multiple vaccines. The co-occurrence of these cardiac adverse events is shown in Table 2. The day of onset for COVID-19 vaccine cardiac adverse events is illustrated in Table 3. The frequency of COVID-19 mRNA vaccine associated myocarditis adverse event differences by dose are shown in Table 4. Figure 1 illustrates myocarditis adverse event for multiple vaccines for males. Differences between adverse event reports by gender are shown in Table 5 for multiple vaccines. Figure 2 illustrates chest pain frequency differences by gender and age.
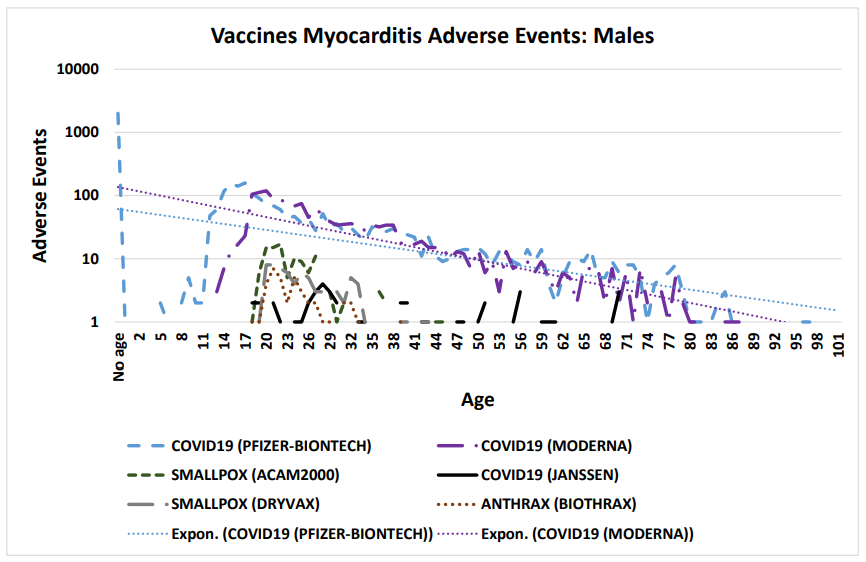
Figure 1: Vaccine associated myocarditis cardiac adverse events in males (COVID-19 Moderna mRNA-1273, COVID-19 Pfizer BNT162b2, COVID-19 Janssen, Anthrax, and Smallpox vaccines)
Discussion
Cardiac adverse events following vaccination can be categorized into the following groups: non-specific (e.g., chest pain and chest discomfort), altered heart rate (arrhythmia, heart rate increased, palpitations, and tachycardia), and cardiac (e.g., myocarditis, pericarditis, and troponin increased). Chest pain is the most frequently reported adverse event (Table 1). The vaccines with the highest numbers of reported cardiac adverse events in VAERS are summarized in Table 1. Consistent patterns across multiple unrelated vaccines suggest a generalized cause that is not vaccine specific (Figure 1 & Table 1). This article proposes that vaccine associated cardiac adverse events scale approximately with the overall reactogenicity level of each vaccine. Co-occurrences of cardiac adverse events are summarized in Table 2. Chest pain occurs with chest discomfort, palpitations, myocarditis, etc. “Troponin increased” is frequently observed with both myocarditis and also chest pain, indicating possible overlapping underlying loss of cardiac myocytes.
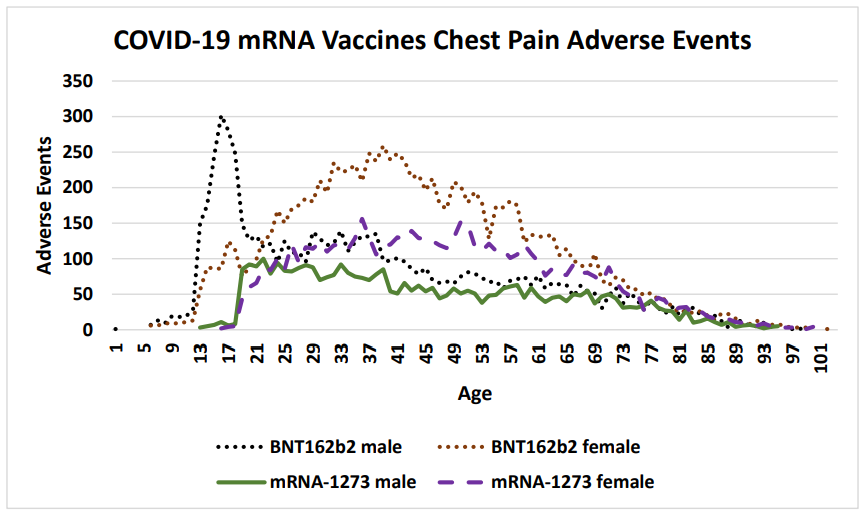
Figure 2: COVID-19 chest pain adverse events by gender and age following SARS-CoV-2 Spike mRNA vaccination reported in the VAERS system by April 1, 2022 (Pfizer BNT162b2 and Moderna mRNA-1273).
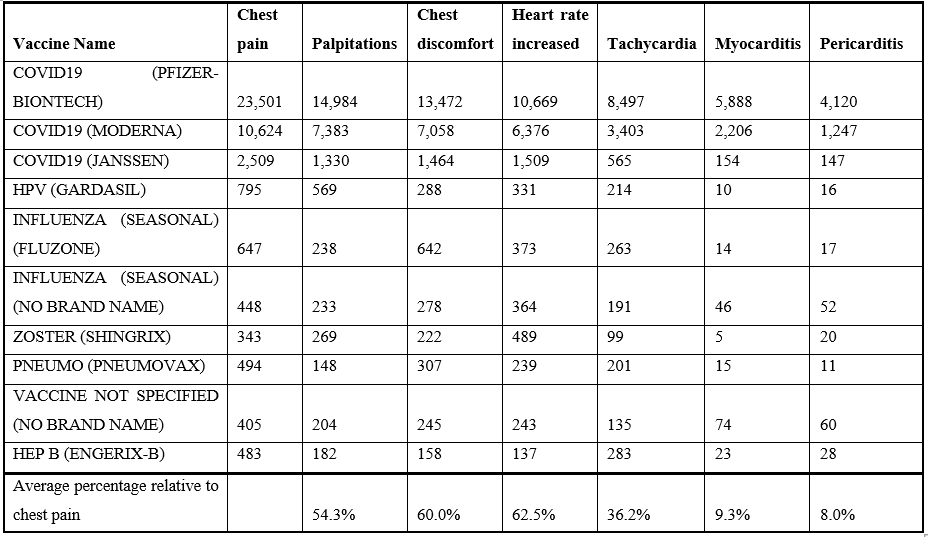
Table 1: Vaccine associated cardiac adverse events from VAERS (1990 to April 1, 2022).
Cardiac adverse events are consistent with altered heart rate and/or predicted cardiac vasoconstrictions. This article proposes that both of these patterns directly result from elevated histamine levels released by innate immune responses to vaccination. The incidence of reported cardiac adverse events is highest within 24 hours and decreases rapidly within days (Table 3). For some cardiac adverse events, the proportion of reported events is lower for the second dose relative to the first dose suggesting possible attenuation from first exposure with the exception of myocarditis with the opposite trend for males (Table 4). For lower second dose incidence frequency, it is possible for histamine metabolism gene(s) from the initial vaccination to be still upregulated for some individuals at the time of administration of the second dose.
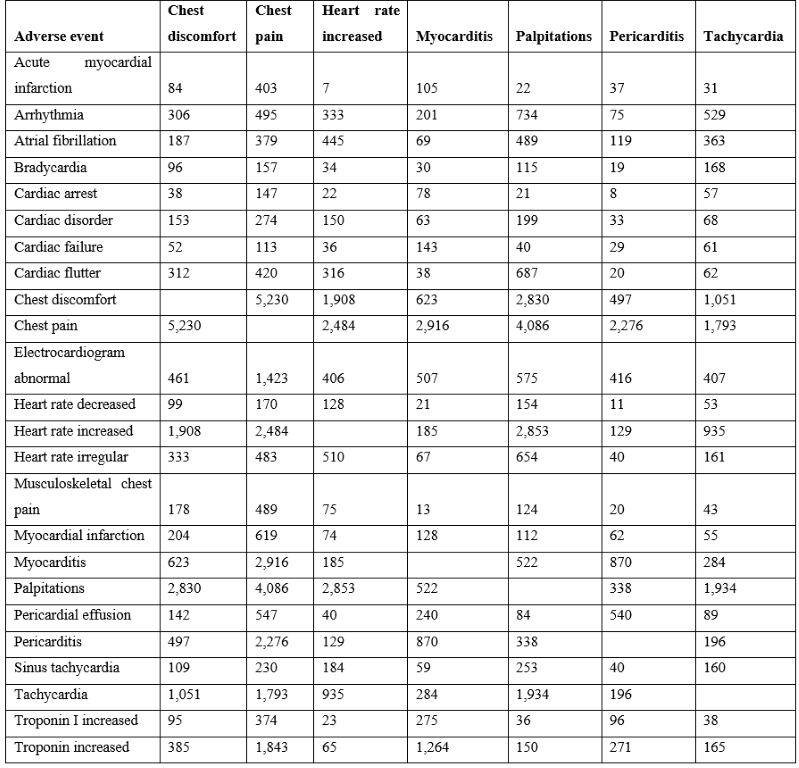
Table 2: Co-occurrences of vaccine associated cardiac adverse events from VAERS (1990 to April 1, 2022).
Vaccinee gender is an important factor for reported cardiac adverse events (Table 5, Figure 2) Immune response differences between genders is known [35–43]. This imbalance is consistent for multiple vaccines except for the anthrax and smallpox vaccines (Table 5); this may be due to imbalanced gender difference in distribution (e.g., military) or other artifact(s). For males, the incidence of reported myocarditis events by age can be modeled by exponential decay patterns (Figure 1) for both Moderna mRNA-1273 and Pfizer BNT162b2 COVID-19 vaccines. While the number of myocarditis reports for both anthrax and smallpox are much lower, a similar pattern decreasing by age might be envisioned. Myocarditis in males may be a function of vaccine reactogenicity coupled to male gender response that decreases with age (Figure 1). A surprising pattern for possible additional myocarditis-like cases may be seen for male teenagers receiving BNT162b2 with the “chest pain” adverse event symptom (Figure 2). During diagnosis, it is important to consider that these cardiac adverse events are being reported for all genders.
Myocarditis has been reported associated with triple vaccination [2]; co-administration of multiple vaccines may increase the amount of histamine release with predicted correspondingly higher frequencies of cardiac adverse events in vaccinees.
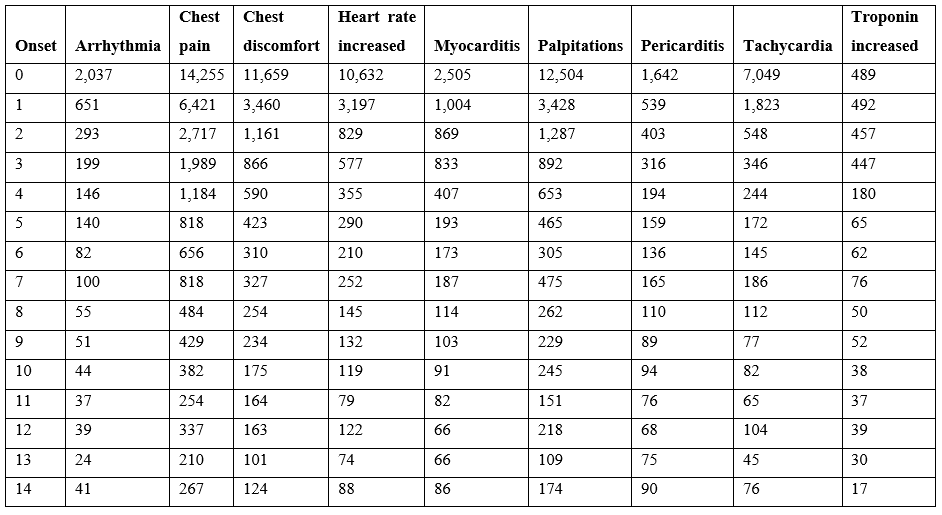
Table 3: COVID-19 vaccine cardiac adverse events onset post vaccination from VAERS up to April 1, 2022.
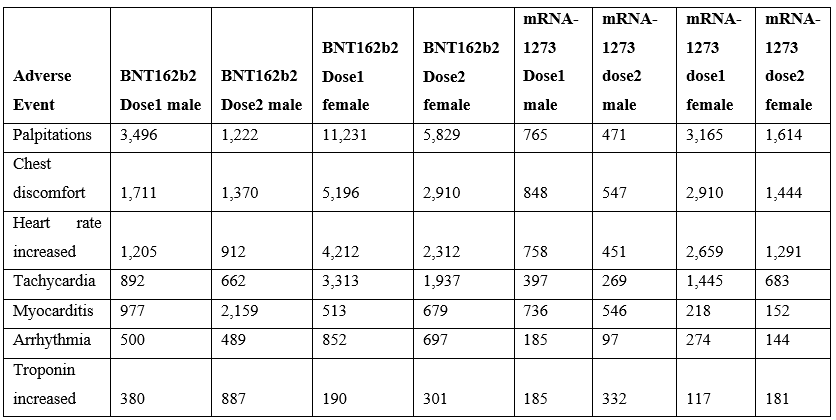
Table 4: Myocarditis by dose following SARS-CoV-2 Spike mRNA vaccination reported in the VAERS system by April 1, 2022 (Pfizer BNT162b2 & Moderna mRNA-1273).
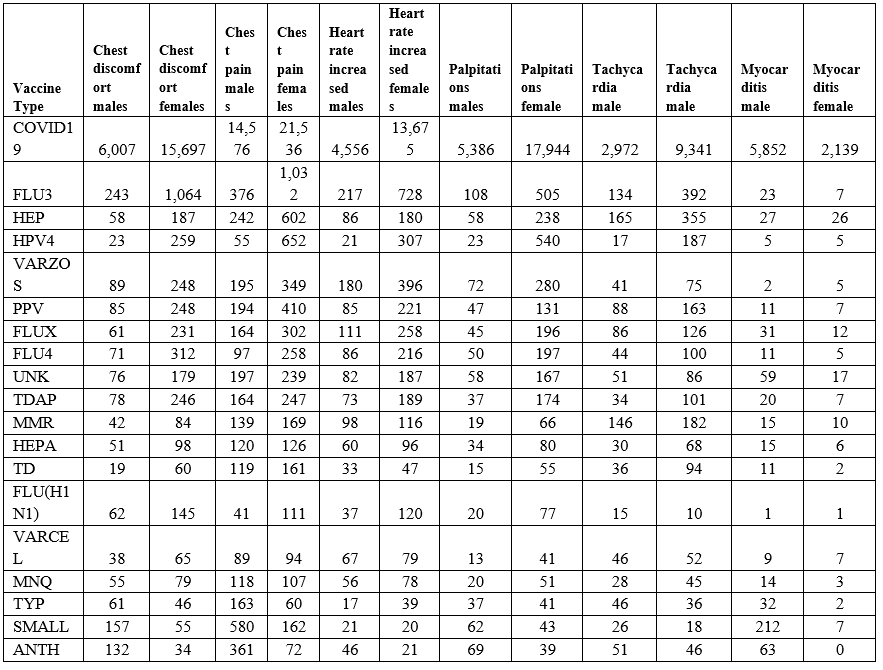
Table 5: Vaccine associated cardiac adverse events gender bias from VAERS from 1990 to April 1, 2022.
Candidate Treatments Suggested by Elevated Histamine Model
The model that most vaccine associated cardiac adverse events are caused by elevated histamine level exceeding an individual’s tolerance level suggests possible combination of prophylactic followed by several days of therapeutic treatments for evaluation in vaccinees. Antihistamine treatments exhibiting efficacy in treating COVID-19 patients are predicted to also target granulocytes and mast cells associated with vaccine responses [44]. These candidate treatments for further evaluation include high dose famotidine [44–47], cetirizine [48,49], and dexchlorpheniramine [48]. Oral treatment with diamine oxidase (DAO) may also minimize or reduce severity vaccine reactogenicity cardiac adverse event symptoms. These treatments may be effective as combined prophylactic and therapeutic treatments for reducing these symptoms. Based on the cardiac symptoms onset patterns observed in Table 3, prophylactic administration prior to vaccination continuing for several days post vaccination would be worth evaluating. Treatment of vaccinees with associated cardiac events may potentially provide symptoms relief while potentially reducing anoxia of cardiac myocyte cells. Evaluation of these treatments and treatment combinations on vaccinees in case reports, case series, etc. clinical studies could drive the design of subsequent randomized controlled clinical trials for reducing vaccine cardiac adverse events. This model and candidate treatments are applicable to multiple vaccines with greater potential benefits for vaccines with higher reactogenicity.
Summary
Elevated histamine levels from innate immune response to vaccination is proposed as causative for associated cardiac adverse events for affected vaccinees; these cardiac adverse events are proposed to occur when the vaccinee’s histamine tolerance level was exceeded. This model predicts that the frequency of cardiac adverse events is related to the reactogenicity level of the vaccine. Specific antihistamines at the proper dosage possibly combined with diamine oxidase may be effective as combined prophylactic and therapeutic treatments in vaccinees with the potential to reduce the incidence rate and severity of cardiac adverse events. Reducing the severity of myocarditis and pericarditis may reduce associated cardiac tissue damage as reflected by troponin levels.
Acknowledgements: None
References:
- Dilber E, Karagöz T, Aytemir K, Özer S, Alehan D, et al. (2003) Acute Myocarditis Associated With Tetanus Vaccination. Mayo Clin Proc. 78: 1431-1433. [PubMed.]
- Amsel SG, Hanukoglu A, Fried D, Wolyvovics M. (1986) Myocarditis after triple immunisation. Arch Dis Child 61: 403-405. [PubMed.]
- Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, et al. (2021) Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 6: 1202. [Ref.]
- Kim HW, Jenista ER, Wendell DC, Azevedo CF, Campbell MJ, et al. (2021) Patients with Acute Myocarditis Following mRNA COVID-19 Vaccination. JAMA Cardiol. 6: 1196. [PubMed.]
- Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, et al. (2021) Symptomatic Acute Myocarditis in 7 Adolescents After Pfizer-BioNTech COVID-19Vaccination. Pediatrics. 148: e2021052478. [Ref.]
- Larson KF, Ammirati E, Adler ED, Cooper LT, Hong KN, Saponara G, et al. (2021) Myocarditis After BNT162b2 and mRNA-1273 Vaccination. Circulation. 144: 506-508. [PubMed.]
- Rosner CM, Genovese L, Tehrani BN, Atkins M, Bakhshi H, et al. (2021) Myocarditis Temporally Associated With COVID-19 Vaccination. Circulation. 144: 502-505. [PubMed.]
- Lane S, Yeomans A, Shakir S. (2021) Reports of myocarditis and pericarditis following mRNA COVID-19 vaccines: A systematic review of spontaneously reported data from the UK, Europe, and the US and of the literature. Pharmacology and Therapeutics). [Ref.]
- Ramírez-García A, Lozano Jiménez S, Darnaude Ximénez I, Gil Cacho A, Aguado-Noya R, et al. (2021) Pericarditis after administration of the BNT162b2 mRNA COVID-19 vaccine. Rev Espanola Cardiol Engl Ed. 74: 1120-1132. [Ref.]
- Das BB, Moskowitz WB, Taylor MB, Palmer A. (2021) Myocarditis and Pericarditis Following mRNA COVID-19 Vaccination: What Do We Know So Far? Children. 8: 607. [PubMed.]
- Pepe S, Gregory AT, Denniss AR. (2021) Myocarditis, Pericarditis and Cardiomyopathy After COVID-19 Vaccination. Heart. 30(10): 1425-1429. [Ref.]
- Hudson B, Mantooth R, DeLaney M. (2021) Myocarditis and pericarditis after vaccination for COVID-19. J Am Coll Emerg Physicians Open. 2: e12498. [Ref.]
- Diaz G, Parsons G, Gering S, Meier A, Hutchinson I, Robicsek A. (2021) Myocarditis and Pericarditis After Vaccination for COVID-19. JAMA. 326: 3. [Ref.]
- Tate C, Demashkieh L, Hakmeh W. (2021) Isolated Tachycardia Presenting After Pfizer-BioNTech COVID-19 Vaccination. Cureus. [Ref.]
- Reddy S, Reddy S, Arora M. (2021) A Case of Postural Orthostatic Tachycardia Syndrome Secondary to the Messenger RNA COVID-19 Vaccine. Cureus. [Ref.]
- Patrignani A, Schicchi N, Calcagnoli F, Falchetti E, Ciampani N, et al. (2021) Acute myocarditis following Comirnaty vaccination in a healthy man with previous SARS-CoV-2 infection. Radiol Case Rep 16: 3321-3325. [PubMed.]
- García MTM, Lana ÁT, Agudo MBA, Delgado MR de la T. (2021) Tachycardia as an undescribed adverse effect to the Comirnaty© vaccine (BNT162b2 Pfizer-BioNTech Covid-19 vaccine): Description of 3 cases with a history of SARS-CoV-2 disease. Enfermedades Infecc Microbiol Clínica. S0213005X21000744. [PubMed.]
- Kaur R, Dutta S, Charan J, Bhardwaj P, Tandon A, et al. (2021) Cardiovascular Adverse Events Reported from COVID-19 Vaccines: A Study Based on WHO Database. Int J Gen Med Volume. 14: 3909-3927. [Ref.]
- Dionne A, Sperotto F, Chamberlain S, Baker AL, Powell AJ, et al. (2021) Association of Myocarditis with BNT162b2 Messenger RNA COVID-19 Vaccine in a BNT162b2 Messenger RNA COVID-19 Vaccine in a Case Series of Children. JAMA Cardiol. 6: 1446. [Ref.]
- Aiba T, Ishibashi K, Hattori K, Wada M, Ueda N, et al. (2021) Frequent Premature Ventricular Contraction and Non-Sustained Ventricular Tachycardia After the SARS-CoV-2 Vaccination in Patient with Implantable Cardioverter Defibrillator Due to Acquired Long-QT Syndrome. Circ J. 85: 2117. [PubMed.]
- Kerneis M, Bihan K, Salem J-E. (2021) COVID-19 vaccines and myocarditis. Arch Cardiovasc Dis. 114: 515-517. [PubMed.]
- Fronza M, Thavendiranathan P, Chan V, Karur GR, Udell JA, et al. (2022) Myocardial Injury Pattern at MRI in COVID-19 Vaccine-associated Myocarditis. Radiology. 212559. [Ref.]
- Husby A, Hansen JV, Fosbøl E, Thiesson EM, Madsen M, et al. (2021) SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 375: e068665. [PubMed.]
- Karlstad Ø, Hovi P, Husby A, Härkänen T, Selmer RM, Pihlström N, et al. (2022) SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents. JAMA Cardiol. [Ref.]
- Levin D, Shimon G, Fadlon-Derai M, Gershovitz L, Sebbag A, et al. (2021) Myocarditis following COVID-19 vaccination - A case series. Vaccine. 39: 6195-200. [Ref.]
- Mevorach D, Anis E, Cedar N, Hasin T, Bromberg M, et al. (2022) Myocarditis after BNT162b2 Vaccination in Israeli Adolescents. N Engl J Med 386: 998-999. [Ref.]
- . Li X, Lai FTT, Chua GT, Kwan MYW, Lau YL, et al. (2022) Myocarditis Following COVID-19 BNT162b2 Vaccination Among Adolescents in Hong Kong. JAMA Pediatr. [Ref.]
- Fremont-Smith M, Gherlone N, Smith N, Tisdall P, Ricke DO. (2021) Models for COVID-19 Early Cardiac Pathology Following SARS-CoV-2 Infection. Int J Infect Dis. 113:331-335. [PubMed.]
- Dale HH, Laidlaw PP. (1910) The physiological action of beta-iminazolylethylamine. J Physiol. 41: 318-344. [PubMed.]
- Skovgaard N, Møller K, Gesser H, Wang T. (2009) Histamine induces postprandial tachycardia through a direct effect on cardiac H 2 -receptors in pythons. Am J Physiol-Regul Integr Comp Physiol. 296: R774-85. [Ref.]
- Wolff AA, Levi R. (1986) Histamine and cardiac arrhythmias. Circ Res. 58: 1-16. [PubMed.]
- Maintz L, Novak N. (2007) Histamine and histamine intolerance. Am J Clin Nutr 85: 1185-1196. [Ref.]
- VAERS. (2021) Vaccine Adverse Event Reporting System. U.S. Department of Health & Human Services. [Ref.]
- Ricke DO. (2022) Hypothesis: Histamine Intolerance Causes Most Major Vaccine Reactogenicity Adverse Events (including SARS-CoV-2 Spike Vaccines). Res Sq. [Ref.]
- Berghöfer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. (2006) TLR7 Ligands Induce Higher IFN-α Production in Females. J Immunol. 177: 2088-2096. [PubMed.]
- Fink AL, Klein SL. (2015) Sex and Gender Impact Immune Responses to Vaccines Among the Elderly. Physiology. 30: 408-416. [Ref.]
- Fink AL, Engle K, Ursin RL, Tang W-Y, Klein SL. (2018) Biological sex affects vaccine efficacy and protection against influenza in mice. Proc Natl Acad Sci. 115: 12477-12482. [PubMed.]
- Fish EN. (2008) The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 8: 737-744. [Ref.]
- Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, et al. (2014) Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Natl Acad Sci. 111: 869-874. [Ref.]
- Klein SL, Flanagan KL. (2016) Sex differences in immune responses. Nat Rev Immunol. 16: 626-638. [Ref.]
- Klein SL, Morgan R. (2020) The impact of sex and gender on immunotherapy outcomes. Biol Sex Differ. 11: 24. [Ref.]
- Morgan R, Klein SL. (2019) The intersection of sex and gender in the treatment of influenza. Curr Opin Virol. 35: 35-41. [Ref.]
- Venkatakrishnan A, Kumar-M P, Silvert E, Garcia-Rivera E, Szenk M, Suratekar R, et al. Female-male differences in COVID vaccine adverse events have precedence in seasonal flu shots: a potential link to sex-associated baseline gene expression patterns n.d. 18. [Ref.]
- Malone RW, Tisdall P, Fremont-Smith P, Liu Y, Huang X-P, White KM, et al. (2021) COVID-19: Famotidine, Histamine, Mast Cells, and Mechanisms. Front Pharmacol. 12: 216. [Ref.]
- Tomera KM, Malone RW, Kittah JK. Hospitalized COVID-19 patients treated with celecoxib and high dose famotidine adjuvant therapy show significant clinical responses. SSRN Prepr n.d. 42. [Ref.]
- Mather JF, Seip RL, McKay RG. (2020) Impact of Famotidine Use on Clinical Outcomes of Hospitalized Patients With COVID-19. Am J Gastroenterol. 115: 1617-1623. [PubMed.]
- Sethia R, Prasad M, Mahapatra SJ, Nischal N, Soneja M, Garg P, et al. (2020) Efficacy of Famotidine for COVID-19: A Systematic Review and Meta-analysis. MedRxiv. 28.20203463. [Ref.]
- Blanco JIM, Bonilla JAA, Homma S, Suzuki K, Fremont-Smith P, et al. (2021) Antihistamines and azithromycin as a treatment for COVID-19 on primary health care - A retrospective observational study in elderly patients. Pulm Pharmacol Ther. 67: 101989-101989. [PubMed.]
- Hogan II RB, Hogan III RB, Cannon T, Rappai M, Studdard J, et al. (2020) Dual-histamine receptor blockade with cetirizine - famotidine reduces pulmonary symptoms in COVID-19 patients. Pulm Pharmacol Ther. 63: 101942. [Ref.]
